Delta S Universe
Moderators: Chem_Mod, Chem_Admin
-
Petrina Kan 2I
- Posts: 102
- Joined: Fri Aug 30, 2019 12:17 am
Delta S Universe
Can the equation 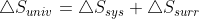 only apply to reversible reactions? Or can it apply to irreversible reactions as well?
only apply to reversible reactions? Or can it apply to irreversible reactions as well?
-
Bryce Barbee
- Posts: 103
- Joined: Wed Sep 18, 2019 12:20 am
Re: Delta S Universe
In a reversible reaction, delta S of the system is equal to delta S of the surroundings because delta S of the universe is 0. But I'm pretty sure that the general equation you described in your question applies to all reactions.
-
Frank He 4G
- Posts: 50
- Joined: Tue Nov 12, 2019 12:19 am
Re: Delta S Universe
That equation can be applied to any situation. It's just telling us that the entropy of the surroundings and the system can be added to get the total change in entropy of the universe. If we're considering an irreversible reaction that happens spontaneously, it will have a positive delta S of the universe.
Return to “Concepts & Calculations Using Second Law of Thermodynamics”
Who is online
Users browsing this forum: No registered users and 3 guests

