Derivation of Boltzwann equation for n moles of gas
Moderators: Chem_Mod, Chem_Admin
-
Cameron Sasmor 1G
- Posts: 11
- Joined: Fri Sep 26, 2014 2:02 pm
Derivation of Boltzwann equation for n moles of gas
I don't understand how kB is changed into nR in the Boltzwann equation for n moles of gas. Can someone explain this please?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Derivation of Boltzwann equation for n moles of gas
All of this assumes two states.
So we have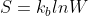 as our equation.
as our equation.
For 1 mole (NA),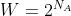
)
We use the log rule to get)
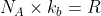 , so we get
, so we get )
This is for one mole, so if we have n moles, we just multiple that by n to get
)
If you have n moles, that's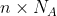 , and plugging that into the equations above and following all the same rules will get you
, and plugging that into the equations above and following all the same rules will get you )
Let's assume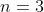
We have)
Then, we use the log rule to get:)
This simplifies to) , where
, where 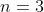 , so we have
, so we have )
So we have
For 1 mole (NA),
We use the log rule to get
This is for one mole, so if we have n moles, we just multiple that by n to get
If you have n moles, that's
Let's assume
We have
Then, we use the log rule to get:
This simplifies to
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Derivation of Boltzwann equation for n moles of gas
I assume you are probably more comfortable with the gas constant R rather than the Boltzmann constant k. These two are really just the same constant, but on different scales.
R has units of J/mol*K and relates to the entropy of 1 mole of particles. R is "macroscopic"
k has units of J/K and relates to the entropy of just 1 particle, so we often say k is "microscopic"
S = k*ln(W) gives the entropy per particle of a system with W states. So Nk*ln(W) is the total entropy of N particles. Dividing N by avogadro's number gives n, the number of moles. Multiplying k by avogadro's number gives R. Since we divided and multiplied by the same number, nothing has changed, and Nk = nR.
R has units of J/mol*K and relates to the entropy of 1 mole of particles. R is "macroscopic"
k has units of J/K and relates to the entropy of just 1 particle, so we often say k is "microscopic"
S = k*ln(W) gives the entropy per particle of a system with W states. So Nk*ln(W) is the total entropy of N particles. Dividing N by avogadro's number gives n, the number of moles. Multiplying k by avogadro's number gives R. Since we divided and multiplied by the same number, nothing has changed, and Nk = nR.
-
Justin Le 2I
- Posts: 142
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Derivation of Boltzwann equation for n moles of gas
I would recommend remembering the log rule where the exponent can be turned into a coefficient. If the equation is left as it is, your calculator might overflow like with one of the homework problems.
Who is online
Users browsing this forum: No registered users and 5 guests

