Page 1 of 1
Using heat capacity to determine molar entropy
Posted: Sat Feb 08, 2020 10:26 am
by Jessa Maheras 4F
The textbook says that you can use heat capacity data to determine the standard molar entropy of a substance and discusses this in 4H.1, but I don't understand how this is done. Can someone explain how or the connection between heat capacity and molar entropy?
Re: Using heat capacity to determine molar entropy
Posted: Sat Feb 08, 2020 10:43 am
by Sarah Zhari 1D
I think that the heat capacity is used in the equation
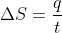
, where the heat capacity is used to calculate the value of q using the equation
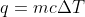
Re: Using heat capacity to determine molar entropy
Posted: Wed Feb 12, 2020 9:53 am
by Jessa Maheras 4F
Sarah Zhari 1D wrote:I think that the heat capacity is used in the equation
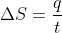
, where the heat capacity is used to calculate the value of q using the equation
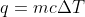
Yes that makes lots of sense thank you! From what I understand now, C=q/mdeltaT , and you can solve for q and use that to find molar entropy? Do you know what about this would make it be at standard molar entropy? (standard in specific?)
Re: Using heat capacity to determine molar entropy
Posted: Wed Feb 12, 2020 10:47 am
by Morgan Carrington 2H
Jessa Maheras 4F wrote:Sarah Zhari 1D wrote:I think that the heat capacity is used in the equation
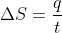
, where the heat capacity is used to calculate the value of q using the equation
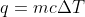
Yes that makes lots of sense thank you! From what I understand now, C=q/mdeltaT , and you can solve for q and use that to find molar entropy? Do you know what about this would make it be at standard molar entropy? (standard in specific?)
I believe that since the q values would have to be held at standard pressure (and/or temperature) in order for the change in entropy to be in standard conditions.