Hi everyone!
I was a little confused about the answer to this question, which states that only NO and N2O would have a residual entropy. Why wouldn't CO2 also have a residual entropy? I remember Dr. Lavelle comparing CO2(g) vs. He(g) during one of the lectures, and he said that CO2(g) would have a higher entropy because the C and O atoms can have different vibrational states contribute to different states. Could you guys explain this to me?
1: On the basis of the structures of each of the molecules, predict which compounds would be most likely to have a residual entropy in their crystalline forms at T=0 K.
NO, Cl2, CO2, N2O
Thank you!
Sapling Weeks 5 and 6 Question #1
Moderators: Chem_Mod, Chem_Admin
-
Mohamed Mido
- Posts: 140
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Sapling Weeks 5 and 6 Question #1
From what I know the reason why CO2 and Cl2 don't have residual entropy is that they have a linear and symmetrical structure. NO and N2O do not have symmetrical structures and there's a possibility for more arrangements. I don't know about Professor Lavelle's comment about CO2 so someone confirm but I think he might have been saying that CO2 has higher entropy relative to Helium since Helium is a monatomic molecule and CO2 obviously isn't, but I don't know if he was making that comment with regard to residual entropy at absolute zero (T=0K).
-
Ven Chavez 2K
- Posts: 101
- Joined: Mon Feb 24, 2020 12:16 am
Re: Sapling Weeks 5 and 6 Question #1
I believe that because at T=0 there is no vibrational entropy which means that the entropy is based on the possible number of orientations. NO, and N2O would have residual entropy because NO can either be N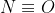 or
or 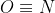 . N2O would also have residual entropy and assuming the N is the central atom, then the second N and O can change swap positions. Cl2 is always Cl-Cl and CO2 always has the carbon as the central atom flanked by two oxygen. Because they are both oxygens, I think swapping them technically is not a different orientation.
. N2O would also have residual entropy and assuming the N is the central atom, then the second N and O can change swap positions. Cl2 is always Cl-Cl and CO2 always has the carbon as the central atom flanked by two oxygen. Because they are both oxygens, I think swapping them technically is not a different orientation.
-
Selena Quispe 2I
- Posts: 129
- Joined: Wed Sep 30, 2020 10:01 pm
- Been upvoted: 3 times
Re: Sapling Weeks 5 and 6 Question #1
The compounds that would have residual entropy are those that are not symmetrical CO2 and Cl2 are perfectly symmetrical so moving them around doesn't cause changes so they will have no entropy. However NO and N20 are not symmetrical, they do not create a perfect crystal structure, and as a result these would have residual entropy
-
Olivia Monroy 1A
- Posts: 107
- Joined: Wed Sep 30, 2020 10:00 pm
Re: Sapling Weeks 5 and 6 Question #1
CO2 and Cl2 (diatomic molecules in general..) are both symmetrical, rearranging the structures doesn't change anything while for the other molecules given there was variation in the structure arrangement which leads to residual entropy.
Re: Sapling Weeks 5 and 6 Question #1
I think NO2 would have residual entropy and not CO2 is because of the molecular arrangement. I'm not sure fully, but I think the 2 equal double bonds in CO2 makes it harder for molecules to have entropy whereas the hybridized state of the NO2 would have more movement and freedom for the electrons to move where they want.
-
VeronicaShepherd3B
- Posts: 101
- Joined: Wed Feb 17, 2021 12:17 am
Re: Sapling Weeks 5 and 6 Question #1
What does it mean to have crystalline form ? What does T stand for?
Re: Sapling Weeks 5 and 6 Question #1
VeronicaShepherd3B wrote:What does it mean to have crystalline form ? What does T stand for?
In this question, T just stand for temperature. The temperature is 0 K which is absolute 0.
Who is online
Users browsing this forum: No registered users and 4 guests

