"During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 C was compressed suddenly (and irreversibly) to 0.500 L by driving in a piston. In the process, the temperature of the gas increased to 28.1 C. Assume ideal behavior. What is the change in entropy of the gas?"
How come when determining the change in volume Cv (5/2 R) is used but when determining the change in temperature just R is used?
Thanks
Homework Question 9.13
Moderators: Chem_Mod, Chem_Admin
-
Isabella Sanzi 2E
- Posts: 54
- Joined: Thu Jul 13, 2017 3:00 am
Re: Homework Question 9.13
So according to what I see in the solution manual, all you have to do is use the formulas deltaS = nRln(V2/V1) and deltaS = nRln(T2/T1), so you don't have to worry about a constant for C at all. Just plug in what is given to solve for the entropy for the whole system based on each step of the reaction taking place. I hope this helps!
-
Lisa Tang 1C
- Posts: 62
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 2 times
Re: Homework Question 9.13
I think you actually mean R is used when volume changes and Cv when temperature changes. The equations in which these values are plugged in are both derived from others. On pages 321 and 323, the derivations are explained for the equation for entropy change of a system with temperature change and one of volume change, respectively.
For a change in temperature, the energy supplied as heat is related to the increase in temperature by the heat capacity, C.
For a change in volume, the equation relates to wrev=-nRT*ln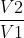 , which is equal to heat released (but with the opposite sign). Using the equation
, which is equal to heat released (but with the opposite sign). Using the equation  S=qrev/T=(-wrev)/T, you can substitute nRT*ln
S=qrev/T=(-wrev)/T, you can substitute nRT*ln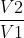 in for -wrev and end up with nR*ln
in for -wrev and end up with nR*ln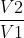 .
.
For a change in temperature, the energy supplied as heat is related to the increase in temperature by the heat capacity, C.
For a change in volume, the equation relates to wrev=-nRT*ln
-
Paula Dowdell 1F
- Posts: 72
- Joined: Tue Nov 15, 2016 3:00 am
Re: Homework Question 9.13
But how do you know that "for a change in temperature, the energy supplied as heat is related to the increase in temperature by the heat capacity, C". Is that always true?
-
Lisa Tang 1C
- Posts: 62
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 2 times
Re: Homework Question 9.13
Paula Dowdell 1F wrote:But how do you know that "for a change in temperature, the energy supplied as heat is related to the increase in temperature by the heat capacity, C". Is that always true?
The definition of heat capacity is the ratio of the heat supplied to the rise of temperature produced: C= q/
Return to “Entropy Changes Due to Changes in Volume and Temperature”
Who is online
Users browsing this forum: No registered users and 11 guests

