When you add more volume, the gas will expand as the entropy always increases. Thus, it is more favorable to fill up the larger volume when possible. It is like there are two people waiting for 1 treadmill, as soon as another treadmill is available, one of them will rush into that treadmill. So the gas will always fill up the volume you give it. Of course as you are increasing the volume of the system, the pressure will drop.
And as the discussion above mention, since entropy is the state function, you just need to calculate
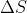
of the reversible process.

