Entropy in reversible and irreversible
Moderators: Chem_Mod, Chem_Admin
-
Diana_Diep2I
- Posts: 130
- Joined: Sat Aug 17, 2019 12:17 am
Entropy in reversible and irreversible
What is the difference in entropy when it comes to a reversible reaction? Irreversible? Do we calculate it the same way using the deltaS=nRln(V2/V1)?
-
nehashetty_2G
- Posts: 102
- Joined: Thu Jul 25, 2019 12:15 am
Re: Entropy in reversible and irreversible
I don't think it matters for entropy. I believe we always use the RTln(V2/V1).
-
Samuel G Rivera - Discussion 4I
- Posts: 61
- Joined: Sat Aug 17, 2019 12:16 am
- Been upvoted: 1 time
Re: Entropy in reversible and irreversible
For reversible you would use delta S = nRln(V2/V1). For isothermal irreversible free expansion you would use the same equation. The difference is that the change in entropy of the surroundings is - delta S for reversible and 0 for irreversible.
-
Abhi Vempati 2H
- Posts: 104
- Joined: Fri Aug 09, 2019 12:17 am
Re: Entropy in reversible and irreversible
Since entropy is a state function and the path between initial and final states doesn't matter, the entropy of the system is nRln(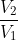 ) for both reversible and irreversible expansion.
) for both reversible and irreversible expansion.
However, as @Samuel G Rivera - Discussion 4I mentioned, the main difference is what happens to the entropy of the surroundings. For reversible expansion (which is typically isothermal),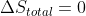 , so
, so 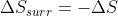 . However, for irreversible expansion, the surroundings don't have time to react to the expansion of the system, so
. However, for irreversible expansion, the surroundings don't have time to react to the expansion of the system, so 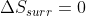 . This means that
. This means that 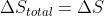 .
.
*Note that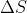 refers to the change in entropy of the system.
refers to the change in entropy of the system.
Hope this helps!
However, as @Samuel G Rivera - Discussion 4I mentioned, the main difference is what happens to the entropy of the surroundings. For reversible expansion (which is typically isothermal),
*Note that
Hope this helps!
Return to “Entropy Changes Due to Changes in Volume and Temperature”
Who is online
Users browsing this forum: No registered users and 9 guests

