Hi,
I'm pretty sure that S> 0 correct, but can the change in entropy or does ΔStot have to be positive? I'm a little confused, thank you!
ΔStotal
Moderators: Chem_Mod, Chem_Admin
Re: ΔStotal
ΔStot for a system depends on the overall changes occurring in the system and its surroundings. If the entropy of the system increases but the entropy of the surroundings decreases by a greater amount the total change in entropy can still be positive. If both the system and the surroundings experience an increase in entropy, total entropy will be positive.
Re: ΔStotal
I think that the second law states that 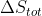 is always greater than 0 since total entropy refers to the entire universe.
is always greater than 0 since total entropy refers to the entire universe.
-
ryuichiro_3I
- Posts: 99
- Joined: Fri Sep 29, 2023 10:59 am
Re: ΔStotal
DeltaS total could be equal to zero if temperature is equal to zero. This also means that delta G is equal to equillbrium.
Return to “Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)”
Who is online
Users browsing this forum: No registered users and 4 guests

