Spontaneous
Moderators: Chem_Mod, Chem_Admin
-
Ian Morris 3C
- Posts: 101
- Joined: Wed Sep 18, 2019 12:18 am
-
Ryan Narisma 4G
- Posts: 104
- Joined: Fri Aug 30, 2019 12:18 am
Re: Spontaneous
Hi Ian Morris 3C! To answer your question, when the Gibbs free energy value is less than 0 (a negative value), the reaction will be spontaneous. However, spontaneity does not correlate with the time it takes for the reaction to complete. I hope this helps!
Re: Spontaneous
well good sir, if the value is negative, it is spontaneous, meaning left over NRG in the form of heat is exuded from the reaction.
-
Catherine Daye 1L
- Posts: 104
- Joined: Wed Sep 11, 2019 12:16 am
-
Eunice Nguyen 4I
- Posts: 100
- Joined: Sat Aug 17, 2019 12:17 am
Re: Spontaneous
In order to determine spontaneity based on Gibbs Free Energy:
ΔG∘<0 = spontaneous
ΔG∘<0 = not spontaneous
ΔG∘<0 = spontaneous
ΔG∘<0 = not spontaneous
-
Justin Sarquiz 2F
- Posts: 106
- Joined: Fri Aug 30, 2019 12:15 am
Re: Spontaneous
Delta G only determines whether a reaction is spontaneous or not. It says nothing about the speed of the reaction.
-
Miriam Villarreal 1J
- Posts: 105
- Joined: Sat Aug 17, 2019 12:16 am
Re: Spontaneous
a spontaneous processes is one that occurs without the addition of external energy. A spontaneous process may take place quickly or slowly, because spontaneity is not related to kinetics or reaction rate.
-
Hailey Kim 4G
- Posts: 110
- Joined: Sat Jul 20, 2019 12:16 am
Re: Spontaneous
A reaction is spontaneous when Delta(G) is greater than 0, or a negative value. When Delta(G) is a positive value, the reaction is not spontaneous, and the reverse reaction is favored. Finally, when Delta(G) is equal to 0, the reaction is at equilibrium.
-
Jainam Shah 4I
- Posts: 130
- Joined: Fri Aug 30, 2019 12:16 am
Re: Spontaneous
Delta G or the change in Gibbs free energy simply states whether a reaction will be spontaneous or not. A spontaneous reaction occurs naturally without input of energy. When Gibbs Free Energy is negative the reaction will be spontaneous
-
Anish Natarajan 4G
- Posts: 51
- Joined: Thu Jul 25, 2019 12:16 am
Re: Spontaneous
If the Gibbs Free Energy is negative--that is, if T*DeltaS is larger than Delta H or Delta H is negative--then the reaction will be spontaneous. The inclusion of T as a variable shows that as temperature increases, all reactions eventually become spontaneous
-
Hussain Chharawalla 1G
- Posts: 100
- Joined: Sat Jul 20, 2019 12:15 am
Re: Spontaneous
It is also useful to note that temperature can affect the free energy in both ways either low or high temperature.
-
Joshua_Chan_3K
- Posts: 46
- Joined: Wed Nov 18, 2020 12:27 am
Re: Spontaneous
Gibbs free energy is negative or less than 0. Going further, this means that the reaction either has a negative change in enthalpy or positive change in entropy. The worth of the change in entropy is also directly related to temperature.
-
Queena Chu 3E
- Posts: 96
- Joined: Wed Sep 30, 2020 10:09 pm
-
Gabriel Nitro 1E
- Posts: 101
- Joined: Wed Sep 30, 2020 9:32 pm
- Been upvoted: 1 time
Re: Spontaneous
Hi,
A reaction is considered spontaneous if Delta G is negative. A nonspontaneous process is where Delta G is positive.
Hope this helps! :)
A reaction is considered spontaneous if Delta G is negative. A nonspontaneous process is where Delta G is positive.
Hope this helps! :)
-
Jason Knight - 1F
- Posts: 113
- Joined: Wed Sep 30, 2020 9:55 pm
Re: Spontaneous
A reaction is spontaneous or not based-on the value of Gibbs free energy. If the value is positive, the reaction is not spontaneous, but if the value is negative, the reaction is spontaneous.
-
Vanshika Bhushan 1A
- Posts: 100
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Spontaneous
When ΔH is negative and ΔS is positive, the sign of ΔG will always be negative, and the reaction will be spontaneous at all temperatures
-
Samantha Lee 1A
- Posts: 108
- Joined: Wed Sep 30, 2020 10:05 pm
- Been upvoted: 1 time
Re: Spontaneous
G < 0 : spontaneous
G > 0 : not spontaneous
This is due to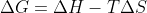
This equation uses the signs and values of H (- or +) , T (in Kelvin so always +), and S (- or +) to get the overall sign and value of G. It G is negative, the reaction is spontaneous, and if G is positive, the reaction is not spontaneous.
* Tip: remember, even if a reaction is spontaneous, that does not mean that the reaction will react quickly; it could be a slow spontaneous reaction.
G > 0 : not spontaneous
This is due to
This equation uses the signs and values of H (- or +) , T (in Kelvin so always +), and S (- or +) to get the overall sign and value of G. It G is negative, the reaction is spontaneous, and if G is positive, the reaction is not spontaneous.
* Tip: remember, even if a reaction is spontaneous, that does not mean that the reaction will react quickly; it could be a slow spontaneous reaction.
-
Jonathan Malau 1F
- Posts: 82
- Joined: Wed Sep 30, 2020 9:31 pm
-
Susanna Givan 2B
- Posts: 144
- Joined: Thu Feb 27, 2020 12:16 am
Re: Spontaneous
what values does enthalpy have to have in order to be spontaneous as compared to nonspontaneous
-
Charmaine Ng 2D
- Posts: 108
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Spontaneous
When deltaG is negative it's spontaneous. It wants to get to a lower free energy state.
Return to “Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)”
Who is online
Users browsing this forum: No registered users and 4 guests

