Gibbs Free Energy
Moderators: Chem_Mod, Chem_Admin
Gibbs Free Energy
Conceptually, what is gibbs free energy? I understand the idea behind entropy, enthalpy, but have no actual understanding what gibbs free energy means on a physical level besides its correlation to spontaneity. Any help?
Re: Gibbs Free Energy
it is a quantity that is used to measure the maximum amount of work done in a thermodynamic system when the temperature and pressure are kept constant in other words the energy available to drive a reaction. which is why it is also said that when del G is 0, max work has been done because all the energy has been utilized. mathematically, 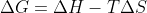 wherein H is enthalpy, S is entropy and T is temperature. this also shows that G depends on these factors. it also tells us if a reaction is spontaneous or not because if del G is negative, reaction is spontaneous whereas if it is positive, it is nonspontaneous and needs an external driving force to occur
wherein H is enthalpy, S is entropy and T is temperature. this also shows that G depends on these factors. it also tells us if a reaction is spontaneous or not because if del G is negative, reaction is spontaneous whereas if it is positive, it is nonspontaneous and needs an external driving force to occur
-
Damin Rawlins 3B
- Posts: 23
- Joined: Mon Jan 09, 2023 9:47 am
Re: Gibbs Free Energy
Gibbs free energy provides information about the spontaneity of a process, which refers to whether a reaction can occur without an input of energy. If the Gibbs free energy change (ΔG) for a process is negative, the process is spontaneous and can occur without an input of energy. If ΔG is positive, the process is non-spontaneous and requires an input of energy to occur. If ΔG is zero, the process is at equilibrium. The physical interpretation of Gibbs free energy is that it represents the amount of energy available to do work. If ΔG is negative, the energy is available to do work and can be harnessed to drive other processes. If ΔG is positive, the energy is not available to do work and cannot be harnessed.
Re: Gibbs Free Energy
Gibbs free energy is essentially the energy in the system that is available to do work. it can also confirm of a reaction is spontaneous or not. when ∆G is negative, it is releasing energy to the surrounding making it spontaneous. when its positive, its non spontaneous since energy is required. only spontaneous reactions can naturally occur.
-
Vanessa Nicoll 3H
- Posts: 34
- Joined: Mon Jan 09, 2023 10:07 am
Re: Gibbs Free Energy
Gibbs free energy is the energy available to do work. Gibbs combines enthalpy and entropy into a single value and we use the change in free energy, delta G, to check its spontaneity. If delta G is positive, it is non spontaneous and an input of external energy is need for the reaction to occur. While, if delta G was negative, it is spontaneous and can occur with external energy.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 11 guests

