Page 1 of 1
Delta G not
Posted: Sun Feb 04, 2018 1:29 pm
by Leah Thomas 2E
Can someone explain the difference between ∆G and ΔG° conceptually. Why don't we always need to calculate for ΔG°?
Re: Delta G not
Posted: Sun Feb 04, 2018 1:51 pm
by Cyianna 2F
It's just a difference of values, the concept of
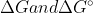
are still the same. I think that it just depends of the conditions of the reaction. I'm not entirely sure though, that's a good question.
Re: Delta G not
Posted: Sun Feb 04, 2018 1:54 pm
by Jason Muljadi 2C
ΔG° is in standard condition, so you would have to calculate ΔH° and ΔS°, just like you would in the regular Gibbs Free Energy equation.
Re: Delta G not
Posted: Sun Feb 04, 2018 1:56 pm
by Jakob von Morgenland 2C
∆G° is considered the standard free energy change of a reaction, while ∆G is considered the free energy change of a reaction. Remember, standard free energy change implies that the reactants and products are in their standard states at 1 atm and generally 25 celsius.
Re: Delta G not
Posted: Sun Feb 04, 2018 3:09 pm
by Ivy Lu 1C
∆G° is the standard free energy of the reaction. This means that the reactants and products are at their standard state: 1 atm for gases and 1 M of aqueous solutions, typically at 25°C. ∆G is just the free energy of the reaction that doesn't have to be under the standard state conditions.
Re: Delta G not
Posted: Sun Feb 04, 2018 3:41 pm
by Diego Zavala 2I
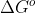
is the standard free energy change in a reaction at 1 bar for gases or 1M for solutions. Because 1 bar is roughly equivalent to 1 atm, we can use the values of the standard free energy for most reactions