enthalpy of sublimation
Moderators: Chem_Mod, Chem_Admin
-
Stephanie tran 1J
- Posts: 50
- Joined: Fri Sep 29, 2017 7:04 am
-
Tasnia Haider 1E
- Posts: 55
- Joined: Sat Jul 22, 2017 3:01 am
Re: enthalpy of sublimation
I'm pretty sure you need to know all the enthalpies, but they should be on the equations sheet.
-
Kyle Alves 3K
- Posts: 46
- Joined: Thu Jul 27, 2017 3:01 am
Re: enthalpy of sublimation
I think the only case it would show up is if we know both the Hvapor and Hsolid, which would make Hsublimation = Hvapor - H solid
and in all cases its endothermic and a positive value!
and in all cases its endothermic and a positive value!
-
Fatima_Iqbal_2E
- Posts: 31
- Joined: Fri Sep 29, 2017 7:06 am
- Been upvoted: 1 time
Re: enthalpy of sublimation
The numerical value should be given to us, but it is important to know the relationship between 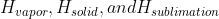 , as stated above!
, as stated above!
-
Rishi Khettry 1L
- Posts: 29
- Joined: Fri Sep 29, 2017 7:05 am
-
Pooja Nair 1C
- Posts: 55
- Joined: Thu Jul 13, 2017 3:00 am
Re: enthalpy of sublimation
As long as you know the equation, delta Hsub = Hvapor - Hsolid, or delta Hsub = delta Hfus + delta Hvap, you should be fine. Just remember that sublimation is changing two states, rather than just one phase transition. Since enthalpy is a state function, you can just add the enthalpy of each phase transition to find the enthalpy of sublimation.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 5 guests

