Calculating Gibbs Free Energy
Moderators: Chem_Mod, Chem_Admin
-
siannehazel1B
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Calculating Gibbs Free Energy
How do we know when to do the difference of sums vs when to use the equation 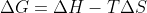 to solve for gibbs free energy?
to solve for gibbs free energy?
-
Lauren Seidl 1D
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Re: Calculating Gibbs Free Energy
You use difference of sums to find Gibbs free energy when you are using the Gibbs free energy of formation for the reactants and products. If you are given entropy, enthalpy, and temperature, then you would use deltaG=deltaH - TdeltaS.
-
RohanGupta1G
- Posts: 34
- Joined: Sat Jul 22, 2017 3:00 am
Re: Calculating Gibbs Free Energy
Usually i look at what information is given and then am able to determine which equation works.
-
Pooja Nair 1C
- Posts: 55
- Joined: Thu Jul 13, 2017 3:00 am
Re: Calculating Gibbs Free Energy
Use difference of sums when the information given pertains to the Gibbs free energy of products or reactants, and the other equation when given entropy and enthalpy. Determine what information the question gives you and then you can choose an equation to plug into accordingly
-
Justin Bui 2L
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
Re: Calculating Gibbs Free Energy
It all depends on what is available to you and then with that, you'd be able to decide what to use. But there's usually a chemical equation or steps when it's a difference of sums situation.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 5 guests

