Predicting Spontaneity
Moderators: Chem_Mod, Chem_Admin
Predicting Spontaneity
For the free energy equation 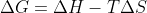 , do we only know how to predict spontaneity simply based on high/low temperatures in part with positive/negative values of
, do we only know how to predict spontaneity simply based on high/low temperatures in part with positive/negative values of 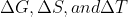 ? Are there any conceptual explanations that we need to know with regards to this?
? Are there any conceptual explanations that we need to know with regards to this?
-
Srikar_Ramshetty 1K
- Posts: 64
- Joined: Fri Sep 28, 2018 12:27 am
Re: Predicting Spontaneity
Well you will want to remember the general rule of thumb of the reaction types, although, not always true. Exothermic reactions tend to be spontaneous since the change in ethalpy is negative and entropy increases. Vice versa for an endothermic reactions. This rule of thumb is helpful in checking your work since there are only a few exceptions. Hope that helps.
-
Angela Cong 3C
- Posts: 69
- Joined: Fri Sep 28, 2018 12:25 am
Re: Predicting Spontaneity
There are charts that you can search up in which it gives you if T is a high number when delta H is positive and so is delta s then it will be spontaneous and so on. It can somewhat help you conceptually. However, essentially if you just plug in big or small values for the equation you can get a gage of whether or not delta H and delta s being positive or negative will have what kind of effect on the system.
Re: Predicting Spontaneity
Yes. We will have to know how to predict spontaneity based on the values.
Sign of the enthalpy change Sign of the entropy change Spontaneity
positive (+) positive (+) The reaction is spontaneous at high temperature
positive (+) negative (-) The reaction is never spontaneous
negative (-) negative (-) The reaction is spontaneous at low temperature
negative (-) positive (+) The reaction is always spontaneous
This table summarizes most of the interactions.
Sign of the enthalpy change Sign of the entropy change Spontaneity
positive (+) positive (+) The reaction is spontaneous at high temperature
positive (+) negative (-) The reaction is never spontaneous
negative (-) negative (-) The reaction is spontaneous at low temperature
negative (-) positive (+) The reaction is always spontaneous
This table summarizes most of the interactions.
-
Cade Okohira 4K
- Posts: 47
- Joined: Fri Sep 28, 2018 12:15 am
Re: Predicting Spontaneity
The Gibbs Free Energy formula can tell about the spontaneity of a rxn based on just the signs of delta H and delta S. If delta H is positive and delta S is negative, then the rxn is not spontaneous, which makes sense because if the enthalpy is positive, this means that the reaction requires heat and delta S being negative shows that entropy decreases, thus going against the usual tendency of a system. If delta H is negative and delta S is positive, then the reaction is spontaneous. If both delta H and delta S are negative or both are positive, the spontaneity depends on the value of the temperature constant.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 11 guests

