Spontaneous vs boiling point?
Moderators: Chem_Mod, Chem_Admin
-
JulieAljamal1E
- Posts: 71
- Joined: Fri Sep 28, 2018 12:24 am
Spontaneous vs boiling point?
In class on Friday, we covered an example that asked what temp Br2(l)->Br2(g) would be spontaneous at 1 ATM? The professor said that this is the same thing as asking what the boiling point is. Can someone explain this? I understand going from a liquid to a gas would involve boiling, but why does that indicate anything about spontaneity?
-
davidbakalov_lec2_2L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:23 am
Re: Spontaneous vs boiling point?
The boiling point is the minimum temperature at which this reaction could be spontaneous. Any temperature higher than the boiling point, the reaction would automatically favor the gas state and be spontaneous.
-
Alexa_Henrie_1I
- Posts: 61
- Joined: Fri Sep 29, 2017 7:03 am
Re: Spontaneous vs boiling point?
It would be the reaction's natural tendency to go from a liquid to a gas at T=boiling point.
-
armintaheri
- Posts: 68
- Joined: Fri Sep 28, 2018 12:26 am
Re: Spontaneous vs boiling point?
A reaction is spontaneous when 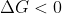
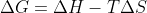
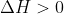 for boiling a liquid, because you are putting heat into the reaction.
for boiling a liquid, because you are putting heat into the reaction.
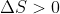 for boiling a liquid, because gases are more disordered than liquids.
for boiling a liquid, because gases are more disordered than liquids.
So to make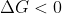 , all you have to do is raise the temperature until
, all you have to do is raise the temperature until 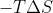 is more negative than
is more negative than  is positive.
is positive.
The boiling point is the temperature at which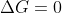 , meaning it changes from positive to negative. So this is the minimum temperature at which the reaction is spontaneous.
, meaning it changes from positive to negative. So this is the minimum temperature at which the reaction is spontaneous.
So to make
The boiling point is the temperature at which
-
Dong Hyun Lee 4E
- Posts: 68
- Joined: Fri Sep 28, 2018 12:23 am
Re: Spontaneous vs boiling point?
Boilin gpoint is when liquid and gas phase coexist so for the example he used would be when T=333k. If T>333K, then a foward process is favored as it was gas phas eonly and the change in s term dominates. And if T<333K then a reverse process is wanted as it is liquid phase only and the change in h term dominates.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 11 guests

