Page 1 of 1
Gibbs Free Energy
Posted: Sun Feb 17, 2019 8:18 pm
by Isabelle Fontanilla 1I
What does it mean when Gibbs Free Energy changes? How does this affect the system?
Re: Gibbs Free Energy
Posted: Sun Feb 17, 2019 9:31 pm
by 505095972
Gibbs free energy is the maximum potential amount of reversible work that can be performed on a thermodynamic system. So a change in this means more or less work can be done on the system which affects the systems change in enthalpy, temperature, and/or change in entropy.
Re: Gibbs Free Energy
Posted: Sun Feb 17, 2019 9:33 pm
by Eva Guillory 2E
Re: Gibbs Free Energy
Posted: Sun Feb 17, 2019 10:59 pm
by lindsey_ammann_4E
Eva Guillory 2E wrote: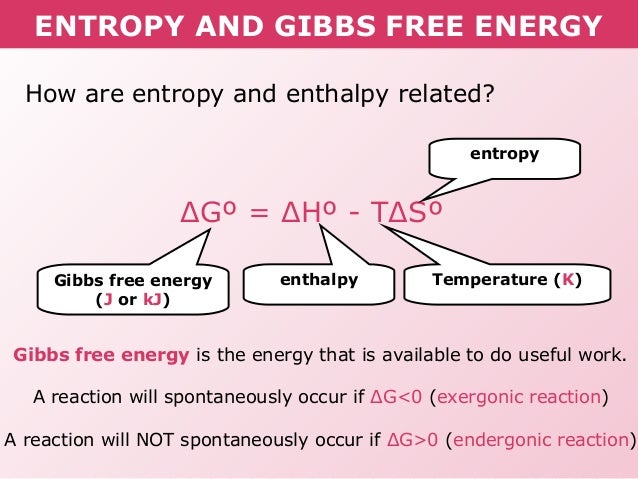
What do exergonic and endergonic mean?
Re: Gibbs Free Energy
Posted: Sun Feb 24, 2019 7:49 pm
by Eva Guillory 2E
lindsey_ammann_4E wrote:Eva Guillory 2E wrote:
What do exergonic and endergonic mean?
In a reaction, exergonic means that energy is released to the surroundings, and endergonic means that energy is absorbed from the surroundings.
Re: Gibbs Free Energy
Posted: Mon Feb 25, 2019 2:42 pm
by Anand Narayan 1G
When a reaction is exergonic, energy is released from the system to the surroundings, and when a reaction is endergonic, energy is taken in (absorbed) from the surroundings
Re: Gibbs Free Energy
Posted: Mon Feb 25, 2019 2:49 pm
by Brian Hom 2F
When Gibbs Free Energy changes, it means that the reaction is more or less favorable. If the change in Gibbs free energy is negative, then the forward reaction is favorable or spontaneous. If the change in Gibbs free energy is positive, then the forward reaction is not favorable. This means that the products have a higher Gibbs free energy state then the reactants, and the goal for compounds is to get to a lower energy state so this is not favorable.
Re: Gibbs Free Energy
Posted: Tue Feb 26, 2019 7:22 pm
by Cade Okohira 4K
Gibbs free energy answers the questions of whether one of the potential driving forces of a chemical reaction are favorable or not. When Gibbs free energy changes from positive to negative, it changes from being not spontaneous to being spontaneous. When it changes from negative to positive, the free energy changes from being spontaneous to not spontaneous, because of the way the equation is structured, with free energy being the enthalpy minus the temperature times the entropy of the reaction.
