∆G = ∆Gº + RT lnQ
Moderators: Chem_Mod, Chem_Admin
-
Ashley Wang 4G
- Posts: 103
- Joined: Wed Sep 11, 2019 12:16 am
∆G = ∆Gº + RT lnQ
Hi,
I'm having trouble understanding the difference between the ∆G and ∆Gº terms in the equation ∆G = ∆Gº + RT lnQ.
Is ∆Gº the standard Gibb's free energy difference between the reactants and products, and ∆G the Gibb's free energy difference between the initial state and equilibrium (since we derived ∆Gº = -RT lnK at equilibrium)? Is this just because we can't assume the reaction is occurring at standard state conditions?
Thank you!
I'm having trouble understanding the difference between the ∆G and ∆Gº terms in the equation ∆G = ∆Gº + RT lnQ.
Is ∆Gº the standard Gibb's free energy difference between the reactants and products, and ∆G the Gibb's free energy difference between the initial state and equilibrium (since we derived ∆Gº = -RT lnK at equilibrium)? Is this just because we can't assume the reaction is occurring at standard state conditions?
Thank you!
-
Leyna Dang 2H
- Posts: 104
- Joined: Thu Jul 25, 2019 12:17 am
Re: ∆G = ∆Gº + RT lnQ
∆G= the change of Gibbs free energy for a system while ∆G°= the change of Gibbs free energy for a system under standard conditions
-
kausalya_1k
- Posts: 50
- Joined: Wed Nov 14, 2018 12:23 am
-
Gerald Bernal1I
- Posts: 102
- Joined: Thu Jul 11, 2019 12:16 am
Re: ∆G = ∆Gº + RT lnQ
This equation relates equilibrium constant with Gibbs free energy. The 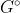 refers to finding the G value when the reactants and products are in their standard states.
refers to finding the G value when the reactants and products are in their standard states.
-
Matthew Tsai 2H
- Posts: 101
- Joined: Wed Sep 18, 2019 12:20 am
Re: ∆G = ∆Gº + RT lnQ
∆Gº refers to change in Gibbs free energy under standard conditions (usually this value will be given)
∆G refers to change in Gibbs free energy for the system (this is usually what we will be calculating for)
∆G refers to change in Gibbs free energy for the system (this is usually what we will be calculating for)
-
sarahforman_Dis2I
- Posts: 109
- Joined: Sat Aug 17, 2019 12:18 am
Re: ∆G = ∆Gº + RT lnQ
Ashley Wang 4G wrote:Hi,
I'm having trouble understanding the difference between the ∆G and ∆Gº terms in the equation ∆G = ∆Gº + RT lnQ.
Is ∆Gº the standard Gibb's free energy difference between the reactants and products, and ∆G the Gibb's free energy difference between the initial state and equilibrium (since we derived ∆Gº = -RT lnK at equilibrium)? Is this just because we can't assume the reaction is occurring at standard state conditions?
Thank you!
An important application of this could be reactions in biological systems.∆Gº and RT will both be constant, but ∆G will not. Like other students have said, ∆Gº is the change in gibs free energy under standard conditions whereas ∆G is the change in gibs free energy for the reaction. Since ∆Gº and RT are constant in biological systems, the reaction quotient Q must be changed in order to make a the ∆G negative. I know this isn't directly relating to your question, but I think it could be a useful application
-
Marni Kahn 1A
- Posts: 107
- Joined: Thu Jul 25, 2019 12:17 am
- Been upvoted: 1 time
Re: ∆G = ∆Gº + RT lnQ
∆G is the change of Gibbs (free) energy for a system and ∆G° is the Gibbs energy change for a system under standard conditions (1 atm, 298K)
-
Renee Grange 1I
- Posts: 56
- Joined: Fri Aug 30, 2019 12:16 am
- Been upvoted: 1 time
-
Brian_Ho_2B
- Posts: 221
- Joined: Fri Aug 09, 2019 12:16 am
Re: ∆G = ∆Gº + RT lnQ
Ashley Wang 4G wrote:Hi,
I'm having trouble understanding the difference between the ∆G and ∆Gº terms in the equation ∆G = ∆Gº + RT lnQ.
Is ∆Gº the standard Gibb's free energy difference between the reactants and products, and ∆G the Gibb's free energy difference between the initial state and equilibrium (since we derived ∆Gº = -RT lnK at equilibrium)? Is this just because we can't assume the reaction is occurring at standard state conditions?
Thank you!
To add on to what others have said, this equation is very important in chemistry and biology because it allows us to relate the delta G at standard conditions with the delta G in general. If we know what the delta G(not) is at standard 1 atm and 298K, we can use the equation to calculate a value for delta G at any given temperature and reaction quotient (any ratio of products to reactants).
-
Katie Bart 1I
- Posts: 104
- Joined: Sat Aug 24, 2019 12:16 am
-
Brian_Ho_2B
- Posts: 221
- Joined: Fri Aug 09, 2019 12:16 am
Re: ∆G = ∆Gº + RT lnQ
Katie Bart 1I wrote:Does the system have to be at equilibrium in order to use this equation?
The system does not have to be at equilibrium or standard conditions to use this equation. The purpose of the "+ RTlnQ" is to account for differences in temperature and concentrations of reactants and products (not at equilibrium). If it was at equilibrium, then Q = K and delta G equals zero, meaning that delta G (not) is equal to "-RTlnK".
-
Sally Qiu 2E
- Posts: 105
- Joined: Fri Aug 30, 2019 12:18 am
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 6 guests

