Page 1 of 1
G vs G knot
Posted: Sun Feb 16, 2020 11:41 pm
by Erik Buetow 1F
Is there any difference between G and G knot or do they represent the same thing?
Re: G vs G knot
Posted: Mon Feb 17, 2020 12:04 am
by Gerald Bernal1I
G knot just means that it is only applicable when the elements in the equation are in their standard state since the knot denotes that it is the Gibbs free energy value at standard state.
Re: G vs G knot
Posted: Mon Feb 17, 2020 12:09 am
by 805422680
G naught is explicitly for elements in their standard state.
Re: G vs G knot
Posted: Mon Feb 17, 2020 12:56 pm
by Alexis Robles 2k
G knot means that the elements in the equation are in their standard state.
Re: G vs G knot
Posted: Mon Feb 17, 2020 12:59 pm
by anjali41
G naught is used for elements in their standard states under normal conditions. G is used for nonstandard conditions.
Re: G vs G knot
Posted: Mon Feb 17, 2020 1:12 pm
by Ashley Tran 2I
This equation also relates G and G

Re: G vs G knot
Posted: Mon Feb 17, 2020 1:17 pm
by Jasmine Vallarta 2L
G knot is under standard conditions
Re: G vs G knot
Posted: Mon Feb 17, 2020 1:34 pm
by Deepika Reddy 1A
G knot indicates that the substances in the equation are all in their standard or most pure states. You will get different answers when solving for either G or G knot but they can be related using the equation mentioned before in another answer.
Re: G vs G knot
Posted: Mon Feb 17, 2020 6:43 pm
by kausalya_1k
G knot is for when everything is in its standard state.
An equation that relates the two terms is deltaG=deltaGknot + RTlnQ
Re: G vs G knot
Posted: Mon Feb 17, 2020 10:06 pm
by Joowon Seo 3A
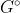
is when the reaction is under standard conditions meaning that the temperature is at 25 C and the pressure is at 1 atm
Regular G is for any temperature and for any pressure
Re: G vs G knot
Posted: Mon Feb 17, 2020 11:11 pm
by 105335337
G Knot is for when the things are at standard state in the world. G is just the G at that specific states.
Re: G vs G knot
Posted: Tue Feb 18, 2020 11:14 am
by Robin Cadd 1D
G is the general term; G knot refers to G under standard state conditions (usually 1 atm and 25 degrees Celsius).
Re: G vs G knot
Posted: Wed Feb 19, 2020 12:38 pm
by Megan Vu 1J
G, or more specifically, delta G, is used in a spontaneous reaction with the energy released that is able to be used as work.
However, delta G is the free energy in a standard state condition.To calculate this, you can use it as a state function by adding or subtracting as a Hess Type approach or from the standard free energy of formation of reactants and products.
Re: G vs G knot
Posted: Wed Feb 19, 2020 1:34 pm
by Renee Grange 1I
Similar to entropy and enthalpy, G knot is G at standard conditions.
Re: G vs G knot
Posted: Wed Feb 19, 2020 2:03 pm
by 205405339
G knot is under standard conditions. G is the total so it will include G knot
Re: G vs G knot
Posted: Wed Feb 19, 2020 3:27 pm
by KaleenaJezycki_1I
Gerald Bernal1L wrote:G knot just means that it is only applicable when the elements in the equation are in their standard state since the knot denotes that it is the Gibbs free energy value at standard state.
This is true, and also normally when referring to equilibrium constants (K) and equilibrium quotients (Q); Gnot is when the system is at equilibrium and G is when the system isn't at equilibrium.