Spontaneity of a System
Moderators: Chem_Mod, Chem_Admin
-
Bronson Mathos 1H
- Posts: 104
- Joined: Wed Sep 30, 2020 9:36 pm
Spontaneity of a System
Hello, I am a bit lost on spontaneity of a reaction and I was wondering if someone could explain how to determine when a reaction is spontaneous in simple terms?
-
Izamary Marquez 2H
- Posts: 107
- Joined: Wed Sep 30, 2020 9:44 pm
Re: Spontaneity of a System
I think the main relationship that we need to know is that if ΔH is negative and –TΔS is positive, the reaction will be spontaneous at low temperatures and if ΔH is positive, and –TΔS negative, the reaction will be spontaneous at high temperatures. Hope this helps!
-
Sara_Lim_2C
- Posts: 106
- Joined: Wed Sep 30, 2020 9:55 pm
Re: Spontaneity of a System
I have a similar problem with remembering which signs are spontaneous at different temperatures, and I definitely recommend looking up a spontaneity chart! Looking at one of those helps me remember the relationships between the different values in formulas.
Re: Spontaneity of a System
It is spontaneous if it results in a less-energetic system. If Gibbs Free Energy for a reaction is negative, meaning that the system loses GFE, then the reaction is "favorable" or "spontaneous"
-
Hayden Lee 1C
- Posts: 104
- Joined: Wed Sep 30, 2020 9:57 pm
Re: Spontaneity of a System
In terms of Gibbs Free Energy, a reaction will be spontaneous depending on the sign of the value of the free energy change. If the value of Gibbs Free Energy is negative, then the reaction is spontaneous. If the value is positive, then the reverse reaction is spontaneous. A negative Gibbs Free Energy value indicates that the reactants have more energy to do work than the products. Therefore, the amount of "free energy" is reduced as the reaction forms products. The opposite is true for a positive value of Gibbs Free Energy change.
-
Mackenzie Stockton 2H
- Posts: 102
- Joined: Wed Sep 30, 2020 10:11 pm
Re: Spontaneity of a System
the Gibbs free energy change of a spontaneous reaction will be negative
g=h-t(delta)S
g=h-t(delta)S
-
Namratha Gujje
- Posts: 106
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Spontaneity of a System
If the change in Gibbs Free energy in a reaction is negative then the reaction is going to spontaneous. You can use the reaction deltaG = delta H - T*delta S to figure out if H and S need to positive or negative for the reaction to be spontaneous.
-
Danielle DIS2L
- Posts: 100
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Spontaneity of a System
In terms of of Gibbs Free Energy, a reaction is spontaneous if the value of the Gibbs Free Energy is negative because it shows that the reactants would have more energy to do work than the products. But if the value is positive, it would be the reverse reaction that would be spontaneous.
-
Xinying Wang_3C
- Posts: 105
- Joined: Wed Sep 30, 2020 9:39 pm
Re: Spontaneity of a System
A reaction is spontaneous when delta G is negative, given the equation delta G=delta H-T*deltaS.
-
Samantha Lee 1A
- Posts: 108
- Joined: Wed Sep 30, 2020 10:05 pm
- Been upvoted: 1 time
Re: Spontaneity of a System
A spontaneous reaction is when  . If
. If  is negative, the reaction will form products spontaneously, without the need for additional energy to be added. Keep in mind that, although the reaction is spontaneous, that does not mean that the reaction is quick, it could be spontaneous and very slow to react. If the reaction is not spontaneous,
is negative, the reaction will form products spontaneously, without the need for additional energy to be added. Keep in mind that, although the reaction is spontaneous, that does not mean that the reaction is quick, it could be spontaneous and very slow to react. If the reaction is not spontaneous, 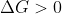 , meaning that it will require extra energy to get the reaction to form products.
, meaning that it will require extra energy to get the reaction to form products.
-
magalysantos_1F
- Posts: 112
- Joined: Wed Sep 30, 2020 9:46 pm
Re: Spontaneity of a System
A spontaneous reaction is a favorable reaction that requires no energy input to create products; the reaction releases more energy as heat as a result. We can usually determine a reaction is spontaneous, for example, if it is exothermic or if it has an increase in DeltaS.
-
Hope Fan 2A
- Posts: 50
- Joined: Wed Nov 18, 2020 12:30 am
Re: Spontaneity of a System
A spontaneous reaction is indicated with a negative delta G, which usually happens when delta H is negative (so exothermic) and/or delta S is positive. A spontaneous reaction is one that proceeds in the forward direction and produces products without any energy input. As for why a spontaneous reaction is indicated with a negative delta G, a negative delta G shows that energy was released as the reaction progresses and so no energy input was required.
-
Gigi Elizarraras 2C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Spontaneity of a System
https://courses.lumenlearning.com/suny-introductory-chemistry/chapter/spontaneity-free-energy-and-temperature/
This chart really helped me understand:) the big picture is just understanding when the gibbs free energy will be negative(neg=spontaneous)
This chart really helped me understand:) the big picture is just understanding when the gibbs free energy will be negative(neg=spontaneous)
-
reyvalui_3g
- Posts: 100
- Joined: Wed Sep 30, 2020 9:52 pm
Re: Spontaneity of a System
We can use the Gibbs free energy equation to figure out if a system is spontaneous. As long as G is less than 0 the reaction will be spontaneous.
-
MCalcagnie_ 1D
- Posts: 86
- Joined: Wed Sep 30, 2020 10:08 pm
Re: Spontaneity of a System
I was confused on this for a while and I finally realized how simple it is recently! A very general thing to remember is that if delta G comes out to be negative, the reaction is spontaneous. If positive, it is not spontaneous.
-
jessicasilverstein1F
- Posts: 134
- Joined: Wed Sep 30, 2020 9:57 pm
-
Carly_Lipschitz_3H
- Posts: 104
- Joined: Wed Sep 30, 2020 9:56 pm
Re: Spontaneity of a System
A reaction is spontaneous when there is a negative gibbs free energy. This means the reaction is favorable. When a reaction is favorable, it is spontaneous, and vice versa.
-
Madison Muggeo 3H
- Posts: 106
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Spontaneity of a System
Hi! In terms of delta G, a system is spontaneous in the forward direction if delta G is negative and spontaneous in the reverse direction if delta G is positive. Hope this helps!!
-
Joel Meza 3I
- Posts: 101
- Joined: Wed Sep 30, 2020 9:56 pm
Re: Spontaneity of a System
In the most simplest terms, when delta G is negative then the reaction is spontaneous and favorable in the forward direction. If delta G is positive, then the reaction is nonspontaneous and favorable in the reverse direction. You can use equations such as " delta G = delta H - T(delta S) " to figure out what the value of delta G is.
-
Mikayla Kwok 3K
- Posts: 109
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Spontaneity of a System
In the most straightforward terms, a reaction is spontaneous when delta G is a negative value. Even if a problem does not explicitly say this, there are ways you can derive this information. For instance, if the problem says that delta H is negative and delta S is positive, you know that delta G is negative based on the equation delta G = delta H - T(delta S). Given that delta G naught is negative or a small positive number, you can also predict that delta G is negative if Q is a very small number less than 1 based on the equation delta G = delta G naught + R*T*(ln Q).
-
Pranav Daggubati 3C
- Posts: 121
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Spontaneity of a System
Generally, a reaction is spontaneous if delta G is negative. Since temperature is always positive because it's in kelvins, it's just a matter of seeing how big either the enthalpy of entropy need to be in order to make delta G negative.
-
Annabella_Amato_1I
- Posts: 105
- Joined: Wed Sep 30, 2020 9:46 pm
Re: Spontaneity of a System
you can determine whether a reaction is spontaneous using the equation ΔG=ΔH−TΔ; if the ΔG is negative, the reaction will be spontaneous because it shows that the reactants had more free energy than the products. If ΔG is positive, the reaction will not be spontaneous for the opposite reason.
-
Ven Chavez 2K
- Posts: 101
- Joined: Mon Feb 24, 2020 12:16 am
Re: Spontaneity of a System
For determining the spontaneity of a reaction, the easiest way to determine it is from the signs of the state functions but mainly Gibbs free energy. If Gibbs free energy is negative then the reaction can be considered spontaneous. If you're given enthalpy and entropy, then by using the equation 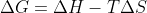 you can determine the sign of G.
you can determine the sign of G.
-
Ivy Tan 1E
- Posts: 100
- Joined: Wed Sep 30, 2020 10:09 pm
- Been upvoted: 2 times
Re: Spontaneity of a System
Hi!
Mathematically, a reaction is spontaneous when delta G is negative. Conceptually, a spontaneous reaction is one that can occur without energy input. For example, a block of ice melting into water at room temperature is spontaneous, but water freezing into ice at room temperature is not spontaneous. Hope this helps!
Mathematically, a reaction is spontaneous when delta G is negative. Conceptually, a spontaneous reaction is one that can occur without energy input. For example, a block of ice melting into water at room temperature is spontaneous, but water freezing into ice at room temperature is not spontaneous. Hope this helps!
Re: Spontaneity of a System
Basically, use the equeation deltaG=deltaH - TdeltaS. The delta G value will tell you if the rxn is spontaneous or not.
-
Sahaj Patel Lec3DisK
- Posts: 130
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: Spontaneity of a System
If delta G (not delta G naught) is negative, then the reaction is spontaneous in the forward direction. If delta G is positive, the reaction is spontaneous in the reverse direction. Hope this helps!
-
MariaCassol1L
- Posts: 49
- Joined: Wed Nov 13, 2019 12:18 am
Re: Spontaneity of a System
The one thing that will give you "true" spontaneity is the Gibbs Free energy of the reaction because it considers both enthalpy and entropy and which temperature that it is occurring in. Remember that elements want to inhabit the lowest energy state possible but want to inhabit more disorderly structures. Therefore enthalpy-wise, reactions that release energy (going from a higher energy state to a lower energy state) are favorable. Entropy wise reactions that go from more ordered states to more disordered states (liquid to gas) are favorable. So to deduce spontaneity you simply need to see if the reaction releases or requires energy and if it has a positive or negative change in entropy (disorder). The Gibbs Free energy equation is a straightforward way to determine which term will dominate depending on temperature because sometimes entropy change will be positive but the reaction will release energy for example.
-
Lauren Sarigumba 1K
- Posts: 102
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Spontaneity of a System
Spontaneity relates to Gibbs Free energy, which helps explain why gas expands to a greater volume when given the opportunity, for example.
-
YuditGaribay3J
- Posts: 50
- Joined: Wed Feb 26, 2020 12:18 am
Re: Spontaneity of a System
I like to picture the diagrams when determining the spontaneity of a system. If it turns out that Gibbs free energy is negative then we would see a fall in potential energy as the reaction progresses and heat is released into the surroundings. I picture the release of heat which makes the potential energy go down which is why it's negative. If it's positive, it is endothermic and will take in heat/energy into the system. This is why the potential energy is high. I like to picture it visually. Hope that makes sense.
-
Tanner Bartyczak 1K
- Posts: 100
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Spontaneity of a System
When the change in Gibbs Free Energy is negative, the reaction is spontaneous.
-
YuditGaribay3J
- Posts: 50
- Joined: Wed Feb 26, 2020 12:18 am
Re: Spontaneity of a System
To follow up with this question, I still have quite a hard time understanding how to use the equation delta G= deltaH=TdeltaS to determine the spontaneity of a reaction using the equation, especially with the temperature. Any tips?
-
Charmaine Ng 2D
- Posts: 108
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Spontaneity of a System
Delta G determines whether a reaction is spontaneous or not, and as other replies have said, you would figure that out based on the equation, DeltaG = DeltaH - (Temperature x DeltaS)
-
Lung Sheng Liang 3J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Spontaneity of a System
A spontaneous reaction is when deltaG is negative and it does not require energy for a reaction to occur. A nonspontaneous reaction is when deltaG is positive and it does require energy for a reaction to occur.
-
Ethan Goode 2H
- Posts: 136
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Spontaneity of a System
It all comes down to the gibbs free energy. With a negative gibbs free energy, a reaction would be spontaneous, and vice versa for positive.
-
ChihWei Chen 2C
- Posts: 58
- Joined: Wed Nov 18, 2020 12:24 am
Re: Spontaneity of a System
You can look at the equation for deltaG: deltaG = deltaH - T*deltaS. Since a reaction is spontaneous when deltaG < 0, you can predict the spontaneity by calculating for deltaH and deltaS and plugging in. You can also predict the spontaneity based on the signs of deltaH and deltaS. If delta H is positive and deltaS is negative, deltaG is always positive, so it is not spontaneous. If deltaH is negative and deltaS is positive, deltaG is always negative, so it is spontaneous.
-
Nan_Guan_1L
- Posts: 106
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Spontaneity of a System
the determinant really is delta G. if Delta G is negative, then the reaction is spontaneous. But you would also need to consider factors that influence the delay G, ie delta H, temperature, and delta S. remember the formula that puts these three together: delta G=delta H -T delta S
-
Hannah Lechtzin 1K
- Posts: 105
- Joined: Wed Sep 30, 2020 9:31 pm
Re: Spontaneity of a System
The spontaneity of a reaction is simply whether or not a reaction is favorable. If you're finding this using the Gibbs Free Energy equation, then a negative delta G indicates that a reaction is spontaneous.
-
FionaHunter21
- Posts: 103
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Spontaneity of a System
If a reaction is spontaneous in one direction is the reverse direction nonspontaneous and vice versa?
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 13 guests

