Non-spontaneous exothermic reaction
Moderators: Chem_Mod, Chem_Admin
-
Fiona Huang 3C
- Posts: 56
- Joined: Wed Nov 25, 2020 12:19 am
Non-spontaneous exothermic reaction
How can an exothermic reaction be nonspontaneous? What would be an example of this?
-
Josh Chou 3K
- Posts: 108
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 3 times
Re: Non-spontaneous exothermic reaction
Combustion reactions are good examples of exothermic reactions that are also nonspontaneous. For example, the combustion of methane with oxygen gas to produce carbon dioxide and water (CH4(g) + 2O2(g) --> CO2(g) + 2H2O(g)) is exothermic because it releases heat, but it is nonspontaneous because it requires an external heat source to proceed with the forward reaction. Using the ΔGf° values in Appendix 2A of the textbook, the sum of the ΔGf° values of the products and reactants is [-50.72 + 2(0)] - [-394.36 + 2(-228.57)] = 800.78. Because ΔGr°is positive, it is nonspontaneous. However, the combustion of methane releases ~890 kJ/mol as heat, and you could calculate the temperature at which this process becomes spontaneous by setting ΔG to 0 and then plugging the ΔH° and ΔS°values of the reaction into the Gibbs free energy equation
-
Kiran Marla
- Posts: 50
- Joined: Wed Nov 18, 2020 12:25 am
Re: Non-spontaneous exothermic reaction
This would occur when the entropy is negative, and the temperature is very high.
-
Jenny Lee 2L
- Posts: 104
- Joined: Thu Oct 08, 2020 12:15 am
Re: Non-spontaneous exothermic reaction
Based on the equation ∆G = ∆H-T∆S, we see there are two factors that make a reaction spontaneous or nonspontaneous, ∆H and ∆S. So if ∆H is negative (in other words the reaction is exothermic), a negative ∆S could make the overall ∆G positive (a nonspontaneous reaction even though exothermic).
-
Keon Amirazodi 3H
- Posts: 107
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Non-spontaneous exothermic reaction
An exothermic reaction can be non-spontaneous when deltaS is negative and the temperature is high.
-
Earl Garrovillo 2L
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
Re: Non-spontaneous exothermic reaction
Spontaneous processes are when ∆G = ∆H-T∆S is negative. In an exothermic process, ∆H is negative but this doesn't always mean ∆G is also negative. If ∆S is negative and the temperature is high enough, ∆G can become positive. I think this is called an entropy-driven reaction or entropy is dominant. In any case, despite the process being exothermic, at the end of the day if ∆G is positive, the process is nonspontaneous.
-
Mari Williams 1K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:53 pm
Re: Non-spontaneous exothermic reaction
An exothermic reaction can be nonspontaneous if the change in entropy is negative and the temperature very high, making it a entropy dominant reaction. Spontaneity of a reaction depends on the sign of delta G, not delta H
-
Emily Vu 1L
- Posts: 106
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 2 times
Re: Non-spontaneous exothermic reaction
i know it's counter-intuitive and i remember dr. lavelle saying non-spontaneous exothermic reactions are sometimes difficult to conceptualize but i would just look at the equation for gibb's free energy and remember that (as what people said above) for delta G < 0, we have to examine enthalpy AND entropy. so a negative delta H for an exothermic reaction cannot be the sole factor for determining spontaneity.
-
MCalcagnie_ 1D
- Posts: 86
- Joined: Wed Sep 30, 2020 10:08 pm
Re: Non-spontaneous exothermic reaction
I was a little confused about this as well, but spontaneity is determined by delta G. So non-spontaneous is when the delta G is positive, but the entropy needs to be negative and temperature is high.
-
Ansh Patel 2I
- Posts: 103
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Non-spontaneous exothermic reaction
Referencing the ∆G = ∆H-T∆S equation, an exothermic reaction can be non-spontaneous when entropy is negative and the temperature is high.
-
SashaAnand2J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Non-spontaneous exothermic reaction
An exothermic reaction may be non-spontaneous if the reaction also leads to a decrease in entropy. Once temperature is above a certain point, this means the reaction becomes entropy-driven, causing the reaction overall to have a positive deltaGo. An online example I found of this is the combustion of methane. Hope that helps!
-
Ranen_Chang_2G
- Posts: 44
- Joined: Wed Nov 25, 2020 12:21 am
Re: Non-spontaneous exothermic reaction
Combustion is a great example. Because it is exothermic,  is negative. To offset this and make
is negative. To offset this and make  , it would require a high T and a negative
, it would require a high T and a negative 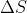
-
Lesly Lopez 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: Non-spontaneous exothermic reaction
It is possible for which an exothermic reaction could be non-spontaneous/ This could happen when  S is negative. Another reason would be that the temperature of the reaction is high.
S is negative. Another reason would be that the temperature of the reaction is high.
-
Gian Boco 2G
- Posts: 96
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Non-spontaneous exothermic reaction
Exothermic reactions only tell the delta H. If the delta S is negative and the temperature value high enough, the delta G can be positive.
-
Grace_Remphrey_2J
- Posts: 100
- Joined: Wed Sep 30, 2020 9:54 pm
Re: Non-spontaneous exothermic reaction
An exothermic reaction can be non-spontaneous when the temperature is high and entropy is negative, as shown in the ∆G = ∆H-T∆S equation.
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 8 guests

