Sapling (5/6) #18
Moderators: Chem_Mod, Chem_Admin
-
Jessica Luong 3K
- Posts: 86
- Joined: Sat Aug 17, 2019 12:17 am
Sapling (5/6) #18
For #18, I have to find the equilibrium constant K using the deltaG(f) equation. I followed all of the instructions where you find the deltaG and then use the equation deltaG = -RT*ln(K). However, I keep getting the wrong answer. Can someone help me with the steps to solve this problem?
Re: Sapling (5/6) #18
One thing that stumped me was that I kept forgetting to convert from kJ to J when calculating for the equilibrium constant. Maybe that could be a place where you miscalculated?
-
Peter DePaul 1E
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Sapling (5/6) #18
So for reaction 1  + 2H_{2}(g)\rightleftharpoons C_{2}H_{6}(g))
-\sum G(Reactants))
 - (209.2 kJ/mol) = -242.0 kJ/mol = -2.420\times 10^{5} J/mol)
After that you substitute into the equation) so we now solve for K
so we now solve for K
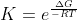 so
so  = 2.5\times 10^{42})
Follow the same process for the other reaction
After that you substitute into the equation
Follow the same process for the other reaction
-
Natalie Do 3F
- Posts: 122
- Joined: Wed Sep 30, 2020 10:03 pm
Re: Sapling (5/6) #18
1. Find G (convert from joules to moles)
ΔG∘r=ΣnΔG∘f(products)−ΣnΔG∘f(reactants)
2. Find K from G
ΔG∘r=−RTln(K)
ΔG∘r=ΣnΔG∘f(products)−ΣnΔG∘f(reactants)
2. Find K from G
ΔG∘r=−RTln(K)
Return to “Van't Hoff Equation”
Who is online
Users browsing this forum: No registered users and 3 guests

