Oxidizing Vs Reducing agent
Moderators: Chem_Mod, Chem_Admin
-
Charlotte Adams 1A
- Posts: 177
- Joined: Wed Sep 30, 2020 9:46 pm
- Been upvoted: 1 time
Oxidizing Vs Reducing agent
Can someone explain what a reducing agent does and what an oxidizing agent does? Also how do you know what is the reducing agent and what is the oxidizing agent when given a chem eqn?
Thanks
Thanks
-
Janelle Gokim 3B
- Posts: 123
- Joined: Wed Sep 30, 2020 9:42 pm
- Been upvoted: 4 times
Re: Oxidizing Vs Reducing agent
The oxidizing agent is what is reduced and the reducing agent is what is oxidized. Think of the the oxidizing agent as the component that allows that oxidation to occur by gaining those lost electrons(reduced) and vice versa.
-
Sabina House 2A
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
- Been upvoted: 4 times
Re: Oxidizing Vs Reducing agent
Just to add on, you can think about how the oxidizing agent (which is the reactant being reduced) is what takes electrons from the reactant being oxidized, allowing this oxidation to occur. The reducing agent (the reactant being oxidized) allows the reduction to occur because it provides electrons to the reactant being reduced. So whichever reactant gains electrons is the oxidizing agent and whichever reactant loses electrons is the reducing agent.
-
Austin Aldujaili 2D
- Posts: 100
- Joined: Wed Sep 30, 2020 9:46 pm
Re: Oxidizing Vs Reducing agent
Whenever it says “agent”, I think of it as the opposite of what it says. So the oxidizing agent would be the reactant being reduced. Another note is that I believe whenever it asks for the agent you want to put in the entire reactant compound as the agent, not just the element/atom that is losing or gaining electrons.
-
Kyla Roche 2K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Oxidizing Vs Reducing agent
Agent basically means opposite here so the oxidizing agent takes the electrons from what is being oxidized therefore it is the in reality the reactant that is being reduced. On the other hand, the reducing agent is what is being oxidized so the reactant that is losing electrons.
-
LarisaAssadourian2K
- Posts: 105
- Joined: Wed Sep 30, 2020 9:34 pm
Re: Oxidizing Vs Reducing agent
I agree with the previous posts. I think of it this way: What's being reduced (gaining electrons) is the oxidizing agent and what's being oxidized (losing electrons) is being reduced. The oxidizing agent takes the electrons and the reducing agent gives the electros.
-
Rajshree 1F
- Posts: 108
- Joined: Wed Sep 30, 2020 9:32 pm
Re: Oxidizing Vs Reducing agent
i try to remember these terms as actual agents. so an oxidizing agent is tasked with oxidizing another molecule and in the process, it gets reduced. a reducing agent is tasked with reducing another molecule and in the process, it gets oxidized. when given an equation, you should first identify the oxidation states of each element on both sides and then write the half reactions.
-
Samudrala_Vaishnavi 3A
- Posts: 103
- Joined: Wed Sep 30, 2020 9:34 pm
Re: Oxidizing Vs Reducing agent
I think of them as opposites of what they actually are if that makes sense. The reducing agent is what is being oxidized, and has a more negative individual standard potential and the oxidizing agent is the molecule that is being reduced (more positive individual standard potential).
-
Madeline Louie 1B
- Posts: 50
- Joined: Sun Nov 15, 2020 12:17 am
Re: Oxidizing Vs Reducing agent
A reducing agent is the one that gets the electrons taken from it and an oxidizing agent is the one that takes the electrons. It also helps to know that a reducing agent is the same as a what gets oxidized and an oxidizing agent is what gets reduced.
-
Melis Kasaba 2B
- Posts: 104
- Joined: Wed Sep 30, 2020 9:49 pm
Re: Oxidizing Vs Reducing agent
The oxidizing agent is the substance that causes oxidation by accepting electrons (thereby getting reduced) and the reducing agent is the substance that causes reduction by losing electrons (thereby getting oxidized).
A helpful tool is OIL RIG: Oxidation is Loss, Reduction is Gain
Here's a useful diagram:
A helpful tool is OIL RIG: Oxidation is Loss, Reduction is Gain
Here's a useful diagram:
-
Aayushi Jani 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:51 pm
Re: Oxidizing Vs Reducing agent
An oxidizing agent is being reduced (it gains electrons and oxidizes another substance) while a reducing agent is being oxidized (since it loses electrons and reduces another substance).
-
Alen Huang 2G
- Posts: 103
- Joined: Wed Sep 30, 2020 9:50 pm
Re: Oxidizing Vs Reducing agent
The oxidizing agent is the one that is oxidizing another species (so oxidizing agent is reduced) and the reducing agent is the one that is reducing another species (so reducing agent is oxidized)
-
Talia Dini - 3I
- Posts: 148
- Joined: Wed Sep 30, 2020 9:32 pm
Re: Oxidizing Vs Reducing agent
The oxidizing agent oxidizes another molecule so it is being reduced and gaining electrons. The reducing agent is reducing another molecule so it is being oxidized and losing electrons.
-
Kristina Krivenko 3I
- Posts: 118
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: Oxidizing Vs Reducing agent
What ___ agent does:
- an oxidizing agent helps another molecule to be oxidized
- a reducing agent helps another molecule to be reduced
What happens to ___ agent itself:
- an oxidizing agent is reduced
- a reducing agent is oxidized
- an oxidizing agent helps another molecule to be oxidized
- a reducing agent helps another molecule to be reduced
What happens to ___ agent itself:
- an oxidizing agent is reduced
- a reducing agent is oxidized
-
YuditGaribay3J
- Posts: 50
- Joined: Wed Feb 26, 2020 12:18 am
Re: Oxidizing Vs Reducing agent
So when we are looking at oxidizing agents it is just the opposites of what we think it is. So normally I think of oxidizing as losing but it is actually gaining? and vise versa?
-
Charlotte Adams 1A
- Posts: 177
- Joined: Wed Sep 30, 2020 9:46 pm
- Been upvoted: 1 time
Re: Oxidizing Vs Reducing agent
YuditGaribay3J wrote:So when we are looking at oxidizing agents it is just the opposites of what we think it is. So normally I think of oxidizing as losing but it is actually gaining? and vise versa?
If you put it that way then yes.
-
Jacob Schwarz-Discussion 3I
- Posts: 111
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Oxidizing Vs Reducing agent
A reducing agent helps another molecule become reduced while an oxidizing agent helps another molecule become oxidized.
In this process, oxidizing agents become reduced and reducing agents become oxidized.
In this process, oxidizing agents become reduced and reducing agents become oxidized.
-
Jarrett Sung 3B
- Posts: 60
- Joined: Wed Sep 30, 2020 9:41 pm
Re: Oxidizing Vs Reducing agent
A reducing agent reduces a reactant while being oxidized itself, and an oxidizing agent oxidizes a reactant while being reduced itself. When looking for each, think about how a reducing agent is oxidized and how an oxidizing agent is reduced.
-
Rachael Cohen 3G
- Posts: 53
- Joined: Sun Dec 13, 2020 12:17 am
Re: Oxidizing Vs Reducing agent
How I like to think about it is that the species that is being oxidized is losing electrons, and those electrons have to go somewhere. They go to another species, causing it to be reduced. So, the species being oxidized is causing the reduction, and is the reducing agent. The opposite is also true - the species that is being reduced is gaining electrons by taking them from another species, thus acting and the oxidizing agent.
-
rita_debbaneh2G
- Posts: 103
- Joined: Wed Sep 30, 2020 9:57 pm
Re: Oxidizing Vs Reducing agent
A reducing agent reduces or donates electrons ( in turn it's the agent is oxidized). ALternatively, the oxidizing agent oxidizes or accepts electrons (in turn being reduced).
-
Evonne Hsu 1J
- Posts: 108
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Oxidizing Vs Reducing agent
So, an oxidizing agent is used to oxidize another molecule. However, in the process, it gets reduced. On the other hand, a reducing agent is used to reduce another molecule, but in the process, it gets oxidized. If you are given an equation in a problem, you should identify the oxidation states of the elements first and then write the half-equations of each.
-
Pranav Daggubati 3C
- Posts: 121
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Oxidizing Vs Reducing agent
An oxidizing agent is an agent or molecule that works to oxidize, or take electrons away, from another molecule. A reducing agent, on the other hand, is one that works to give electrons to another molecule.
An example of a redox reaction in action will give this proper context: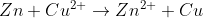
Here, the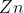 is being oxidized by the oxidizing agent
is being oxidized by the oxidizing agent 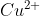 and being oxidized into
and being oxidized into 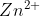 . At the same time, the
. At the same time, the 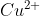 is being reduced by the reducing agent
is being reduced by the reducing agent 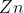 and being reduced into
and being reduced into 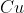
An example of a redox reaction in action will give this proper context:
Here, the
Re: Oxidizing Vs Reducing agent
The oxidizing agent is a molecule that is reduced (gains electrons) in the process of taking electrons away and oxidizing another molecule.
The reducing agent will do the opposite, oxidized (loses electrons) in the process of giving them to another molecule and reducing it
You can tell which molecule is an oxidizing or reducing agent by seeing if they lost electrons (reducing agent) or gained them (oxidizing agent.)
The reducing agent will do the opposite, oxidized (loses electrons) in the process of giving them to another molecule and reducing it
You can tell which molecule is an oxidizing or reducing agent by seeing if they lost electrons (reducing agent) or gained them (oxidizing agent.)
-
Neha Jonnalagadda 2D
- Posts: 110
- Joined: Fri Sep 24, 2021 7:06 am
Re: Oxidizing Vs Reducing agent
A reducing agent is an element or compound that loses or "donates" an electron to an electron recipient in a redox chemical reaction. Oxidizing agent is a substance that has the ability to oxidize other substances — in other words to accept their electrons.
-
Ruirui Lan
- Posts: 50
- Joined: Mon Jan 03, 2022 9:15 pm
Re: Oxidizing Vs Reducing agent
Hi! we can illustrate this using an example: Mg+ Br2 = MgBr2. In this example, the oxidation state of Mg went from 0 to +2, and the oxidation state of Br went from 0 to -1. For Mg, it was oxidized, and so it is the reducing agent. Br was reduced, so it's the oxidizing agent. Oxidation is the losing of electrons while reduction is the gaining of electrons. An oxidizing agent gains electrons, so it's reduced, so in this case is Br. An reducing agent loses electrons, so it's Mg.
-
Trisha Badjatia 2L
- Posts: 109
- Joined: Fri Sep 24, 2021 6:54 am
Re: Oxidizing Vs Reducing agent
Hi! The way I like to think about is that a reducing agent is oxidized, while the oxidizing agent is reduced. Because the reducing agent is just another way of saying that substance is being oxidized, just find the substance that is losing electrons (e.g., getting oxidized). Similarly, the oxidizing agent is getting reduced so find the substance that is gaining negative charge. Simply put, the reducing agent is supplying electrons to the oxidizing agent. Hope that helps!
-
Katherine Li 1A
- Posts: 110
- Joined: Fri Sep 24, 2021 6:38 am
Re: Oxidizing Vs Reducing agent
Recalling back to OIL RIG, you should remember that things lost electrons when oxidized and gain electrons when reduced. An oxidizing agent, however, is not the thing that is oxidizes; rather, it is the agent the oxidizes the other reactant. This means that an oxidizing agent is actually being reduced, as it is taking the electrons from the other reactant. Similarly, a reducing agent is being oxidized.
-
Aanjaneyaa
- Posts: 51
- Joined: Tue Feb 02, 2021 12:17 am
Re: Oxidizing Vs Reducing agent
The oxidizing agent gains the electron. The reducing agent loses the electron.
-
Vincent Real 2C
- Posts: 43
- Joined: Mon Jan 03, 2022 8:38 pm
Re: Oxidizing Vs Reducing agent
An oxidizing agent is a chemical compound/molecule that receives electrons in a redox reaction. On the other hand, a reducing agent is a chemical compound/molecule that donates electrons to the oxidizing agent in a redox reaction.
-
Skylar Lo 2C
- Posts: 110
- Joined: Fri Sep 24, 2021 5:10 am
Re: Oxidizing Vs Reducing agent
The oxidizing agent is what is being reduced and the reducing agent is what is being oxidized. Their swapped around because its the effect on the other.
-
Diana Avalos
- Posts: 55
- Joined: Tue Feb 16, 2021 12:15 am
Re: Oxidizing Vs Reducing agent
OIL RIG, ---> oxidation is loss of electron and reduction is gain of an electron. A reducing agent losses an electron to give to the other compound/ molecule, while the oxidizing agent gains an electron from a compound/ molecule.
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
Re: Oxidizing Vs Reducing agent
an oxidizing agent is the thing that is being reduced while a reducing agent is basically the thing being oxidized so essentially they are kind of opposites.
-
Michelle Argueta 1E
- Posts: 103
- Joined: Fri Sep 24, 2021 6:53 am
Re: Oxidizing Vs Reducing agent
In the acronym of OIL RIG, it states that molecules lose electrons when oxidized and gain electrons when reduced. However, an oxidizing agent is actually being reduced (gains electrons)taking the electrons from the other reactant and a reducing agent is being oxidized (donating electrons).
Re: Oxidizing Vs Reducing agent
An oxidizing agent is an agent that gains electrons and is reduced in a chemical reaction, while a reducing agent loses electrons and is oxidized in a chemical reaction. A reducing agent is typically in one of its lower possible oxidation states, while an oxidizing agent is in one of its higher possible oxidation states.
-
Natalie Coughlin 1I
- Posts: 50
- Joined: Mon Jan 03, 2022 10:57 am
Re: Oxidizing Vs Reducing agent
The oxidizing agent gains electrons to bring about oxidation, while the reducing agent loses electrons to bring about reduction.
-
Neha Mukund
- Posts: 109
- Joined: Fri Sep 24, 2021 5:23 am
Re: Oxidizing Vs Reducing agent
In a reaction, the oxidizing agent is what is reduced and causes the oxidization of another molecule. The reducing agent is what is oxidized and causes the reduction of another molecule. Oxidation is the loss of electrons, whereas reduction is the gain of electrons.
-
Ruben Adamov 1E
- Posts: 105
- Joined: Fri Sep 24, 2021 5:56 am
Re: Oxidizing Vs Reducing agent
The oxidizing agent is being reduced and the reducing agent is being oxidized. Think of the opposite when you see “agent”
-
Kaitlyn Bateman 1L
- Posts: 100
- Joined: Fri Sep 24, 2021 6:52 am
Re: Oxidizing Vs Reducing agent
The oxidizing agent is what allows the other material to be oxidized, thus it itself is reduced. The reducing agent allows the other molecule to be reduced, and it is oxidized. It is a confusing phrase and definition.
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
Re: Oxidizing Vs Reducing agent
an oxidizing agents is reduced in the reaction, and oxidizes the other reagent, a reducing agent is oxidized during the reaction, while it reduces the other reactant.
-
Veronica Larson- 1I
- Posts: 103
- Joined: Wed Nov 11, 2020 12:18 am
Re: Oxidizing Vs Reducing agent
The "agent" is going to be the substance that does the oxidizing or reducing, so therefore it will be the opposite of what is in the word. For example, the oxidizing agent is going to be the substance that is reduced, because the fact that it is reduced means it is doing the oxidizing.
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 5 guests

