Balancing Redox Reaction with Hydrogens
Moderators: Chem_Mod, Chem_Admin
-
Kelly Tran 3N
- Posts: 11
- Joined: Sat Jul 09, 2016 3:00 am
Balancing Redox Reaction with Hydrogens
Why do some redox reactions involve hydrogen protons as well? For example, in problem 14.1.b and 14.1.c, the half reactions are balanced with both hydrogen protons and electrons.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Balancing Redox Reaction with Hydrogens
When balancing redox reactions, you always use 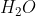 to balance out the oxygens. In acidic conditions, you would then use H+ to balance the hydrogens in the reaction.
to balance out the oxygens. In acidic conditions, you would then use H+ to balance the hydrogens in the reaction.
-
Jordan Pace 2B
- Posts: 14
- Joined: Wed Sep 21, 2016 2:57 pm
Re: Balancing Redox Reaction with Hydrogens
My TA gave us a list of steps for how to balance redox reactions!
Step 1: Write the skeletons of the half-reactions
Step 2: Balance all elements other than H and O
Step 3: Balance O by adding H2O to the side without O
Step 4: Balance H by adding H+ to the side without H
Step 5: Balance charges by adding e- (electrons)
Step 6: Balance overall charges of both half-reactions by multiplying by a common factor
Step 7: Add the two reactions
-If acidic, stop here
-If basic, continue to step 8
Step 8: Add OH- to both sides to cancel H+ ions
Step 9: Combine OH- and H+ to form H2O
Step 10: Cancel H2O molecules and check charge balance
Hope this helps!
Step 1: Write the skeletons of the half-reactions
Step 2: Balance all elements other than H and O
Step 3: Balance O by adding H2O to the side without O
Step 4: Balance H by adding H+ to the side without H
Step 5: Balance charges by adding e- (electrons)
Step 6: Balance overall charges of both half-reactions by multiplying by a common factor
Step 7: Add the two reactions
-If acidic, stop here
-If basic, continue to step 8
Step 8: Add OH- to both sides to cancel H+ ions
Step 9: Combine OH- and H+ to form H2O
Step 10: Cancel H2O molecules and check charge balance
Hope this helps!
Re: Balancing Redox Reaction with Hydrogens
Jordan's post is extremely helpful! This should definitely be endorsed! :)
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 6 guests

