Acidic vs basic solutions
Moderators: Chem_Mod, Chem_Admin
-
Hannah Yates 1K
- Posts: 59
- Joined: Fri Sep 28, 2018 12:27 am
-
amogha_koka3I
- Posts: 62
- Joined: Fri Sep 28, 2018 12:24 am
Re: Acidic vs basic solutions
For balancing half reactions in both acidic and basic solutions, first balance the elements that are not Hydrogen or Oxygen. Then balance the O atoms by adding H20. For the next step: in acidic solution, balance H by using H+. But In basic solution, balance H by using H20 to the side of each half reaction that needs H and adding OH to the other side.
Re: Acidic vs basic solutions
I'm assuming you are referring to balancing redox reactions in acidic or basic conditions.
When you are balancing equations, you want to make sure that the same amount of atoms are on both sides of the equation right? This applies to hydrogen as well, and when you are balancing redox reactions that involve Oxygen and/or hydrogen in the species being oxidized/reduced, there are a few ways we can go about doing this.
In acidic solutions, we know that H+ is readily available and so if we need to have more hydrogen on one side of a reduction or oxidation, we can just add H+ to that side.
e.x:
1) staring with in acidic solution
in acidic solution
2) add 2 H2O to the right side in order to balance out the 2 O atoms in MnO2:
3) now add 4H+ to the left side in order to balance out the 4H atoms in 2H2O: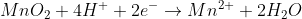
4) Check the final equation and now you see that the number of atoms and total charge on each side is equal.
In basic solutions, we know that OH- ions are readily available. For every hydrogen you need on one side of a reaction to balance out H, you add one H2O to that side and one OH- on the other side. Since the difference between OH- and H2O is one H atom, doing this is the equivalent of adding one H atom to the side of the reaction that you need H atoms.
e.x:
1) starting with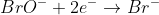 in basic solution
in basic solution
2) add one H2O to the right side to balance out O in BrO-: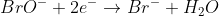
3) To balance out H, add 2HO- to the right side and 2H2O on the left side: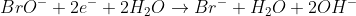
4) The last step results in the left side having 4 more H than before, and now both sides are balanced. However, we can cancel out the H2O on the right, which gives us: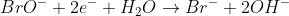
5) Check the final equation and you will see that The O, H, and total charge on each side is now equal.
When you are balancing equations, you want to make sure that the same amount of atoms are on both sides of the equation right? This applies to hydrogen as well, and when you are balancing redox reactions that involve Oxygen and/or hydrogen in the species being oxidized/reduced, there are a few ways we can go about doing this.
In acidic solutions, we know that H+ is readily available and so if we need to have more hydrogen on one side of a reduction or oxidation, we can just add H+ to that side.
e.x:
1) staring with
2) add 2 H2O to the right side in order to balance out the 2 O atoms in MnO2:
3) now add 4H+ to the left side in order to balance out the 4H atoms in 2H2O:
4) Check the final equation and now you see that the number of atoms and total charge on each side is equal.
In basic solutions, we know that OH- ions are readily available. For every hydrogen you need on one side of a reaction to balance out H, you add one H2O to that side and one OH- on the other side. Since the difference between OH- and H2O is one H atom, doing this is the equivalent of adding one H atom to the side of the reaction that you need H atoms.
e.x:
1) starting with
2) add one H2O to the right side to balance out O in BrO-:
3) To balance out H, add 2HO- to the right side and 2H2O on the left side:
4) The last step results in the left side having 4 more H than before, and now both sides are balanced. However, we can cancel out the H2O on the right, which gives us:
5) Check the final equation and you will see that The O, H, and total charge on each side is now equal.
-
Diana Bibireata 1B
- Posts: 60
- Joined: Fri Sep 28, 2018 12:23 am
Re: Acidic vs basic solutions
For acidic solutions you balance out the oxygens by adding H2O and you balance the hydrogens by adding H+.
For basic solutions you balance the oxygens by adding H2O and then you balance the hydrogens by adding H2O to the side that requires more H atoms and add the same amount of OH- to the other side.
For basic solutions you balance the oxygens by adding H2O and then you balance the hydrogens by adding H2O to the side that requires more H atoms and add the same amount of OH- to the other side.
-
Edward Suarez 1I
- Posts: 75
- Joined: Fri Sep 28, 2018 12:27 am
Re: Acidic vs basic solutions
for basic solutions, you use OH- and for acidic solutions you use H+ for balancing purposes
-
George Ghaly 2L
- Posts: 66
- Joined: Fri Sep 28, 2018 12:29 am
- Been upvoted: 1 time
Re: Acidic vs basic solutions
Both are balanced by adding water molecules to a the reactants or products, but for acidic reactions, they are balanced with H^+ while basic reactions are balanced by adding hydroxides to a side.
Re: Acidic vs basic solutions
For acidic solutions you balance the hyrdrogen by adding H+ and for basic solutions you balance the hydrogen by adding OH-.
-
Aili Ye 4L
- Posts: 58
- Joined: Fri Sep 28, 2018 12:16 am
Re: Acidic vs basic solutions
You can balance both initially using H+, but if you are balancing in a basic solution you must add OH- to neutralize the H+
-
Jasmine Chow 1F
- Posts: 60
- Joined: Fri Sep 28, 2018 12:16 am
- Been upvoted: 1 time
Re: Acidic vs basic solutions
A acidic solution had a greater abundance of hydrogen ions making the solution acidic while a basic solution will have a greater abundance of hydroxyl (OH-).
-
LedaKnowles2E
- Posts: 62
- Joined: Fri Sep 28, 2018 12:27 am
Re: Acidic vs basic solutions
In acidic solutions, balance half-reactions by adding H2O and H+; in basic solutions, balance by adding H2O and OH-.
-
Jack Hewitt 2H
- Posts: 67
- Joined: Fri Sep 28, 2018 12:27 am
Re: Acidic vs basic solutions
For an acidic solution you would use H20 and H+ to balance the hydrogens and oxygens while in a basic solution you would use H20 and OH- to balance the hydrogens and oxygens.
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 5 guests

