Redox Table
Moderators: Chem_Mod, Chem_Admin
Redox Table
If all of the reactions in the table represent reductions, for the oxidizing half reaction, it would be made negative, then would the sign in the Ecat-Eanode change to addition or would we subtract it?
-
TimVintsDis4L
- Posts: 104
- Joined: Sat Aug 17, 2019 12:17 am
Re: Redox Table
If you are asking whether or not to change the sign when subtracting the anode from cathode then remember this simple rule.
When you are doing E(cell) = E(cathode) - E(anode), then you use the numbers given as is, and DO NOT change the sign based on whether its Oxidized or not.
For example
If your reactions are
Reduction :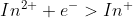 = -.40V
= -.40V
Oxidation :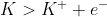 = -2.93V
= -2.93V
Although the appendix gives the oxidation in the form of a reduction, you do not need to flip the sign like you might think. Instead leave it and subtratc
E= -.40V - (-2.93V)
Were you to flip the sign, then you would want to add the numbers
E= -.40V + (+2.93V)
Ultimately, you would get the same answer.
When you are doing E(cell) = E(cathode) - E(anode), then you use the numbers given as is, and DO NOT change the sign based on whether its Oxidized or not.
For example
If your reactions are
Reduction :
Oxidation :
Although the appendix gives the oxidation in the form of a reduction, you do not need to flip the sign like you might think. Instead leave it and subtratc
E= -.40V - (-2.93V)
Were you to flip the sign, then you would want to add the numbers
E= -.40V + (+2.93V)
Ultimately, you would get the same answer.
-
Gabriella Bates 2L
- Posts: 113
- Joined: Thu Jul 11, 2019 12:15 am
Re: Redox Table
You can think about it both ways. If you keep the sign of Eanode as it is, then Ecell = Ecathode - Eanode. However, if you flip the sign of Eanode since it is an oxidation, then Ecell = Ecathode + Eanode. Just be clear which method you use and you will be fine!
-
Sjeffrey_1C
- Posts: 108
- Joined: Wed Feb 20, 2019 12:17 am
Re: Redox Table
The equation takes into consideration that the values are reduction potential values, even though half of the reaction is actually in oxidation. Thus, you don't need to convert the values to oxidation potential.
Re: Redox Table
If you already flip the value for the oxidation part of the reaction, then you just have to add them, or you can subtract the cathode from the anode for both reduction values. Either way it is the same, but it can get confusing.
-
Eugene Chung 3F
- Posts: 142
- Joined: Wed Nov 15, 2017 3:03 am
Re: Redox Table
smurphy1D wrote:If all of the reactions in the table represent reductions, for the oxidizing half reaction, it would be made negative, then would the sign in the Ecat-Eanode change to addition or would we subtract it?
If you don't want to worry about changing the sign, it's easier to just do Ecat-Eanode and always consider the red. rxn for both anode and cathode.
-
Althea Zhao 1B
- Posts: 101
- Joined: Mon Jun 17, 2019 7:24 am
Re: Redox Table
The equation for E(cell) allows you to use the given reduction potentials for each half-reaction: making E(anode) a negative value accounts for the oxidation sign change.
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 14 guests

