oxidation numbers
Moderators: Chem_Mod, Chem_Admin
-
Pratika Nagpal
- Posts: 48
- Joined: Wed Feb 19, 2020 12:20 am
Re: oxidation numbers
you typically look at elements that pretty much have constant oxidation numbers (like O being 2-, the only exception being when in H2O2, and H being +1) and then calculate the oxidation numbers of other elements in the compound based on what charge of the overall compound. ie, with Mn2O7, the charge is neutral and each O has a charge of -2, so Mn is +7 to counter the aggregate -14 charge of the O atoms.
-
Valerie Tran 2B
- Posts: 105
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 2 times
Re: oxidation numbers
https://socratic.org/questions/how-do-y ... a-compound
This is a really good website with examples as well. Hope this is helpful to you!
This is a really good website with examples as well. Hope this is helpful to you!
-
Emily Jacobo 1C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:37 pm
Re: oxidation numbers
I had the same question, wanted to make sure i was not calculating them incorrectly tomorrow. Thank you to those that commented for the steps and helpful tips!
-
Jeremy Wei 2C
- Posts: 103
- Joined: Wed Sep 30, 2020 9:59 pm
-
rita_debbaneh2G
- Posts: 103
- Joined: Wed Sep 30, 2020 9:57 pm
Re: oxidation numbers
There are some that you would just know with experience and memorization (like H and Cl for example) and others that change given the compound that they're in. Also, elements that are just on their own would be zero. Also, all the oxidation numbers in a compound must add up to equal zero (unless it's a polyatomic ion in which they must add up to that charge).
-
Nathan Tong 3G
- Posts: 101
- Joined: Wed Sep 30, 2020 9:49 pm
Re: oxidation numbers
Right, just keep in mind those elements that have special rules. Make sure to double check that all the oxidation numbers add up in the end.
-
Pranav Daggubati 3C
- Posts: 121
- Joined: Wed Sep 30, 2020 9:35 pm
Re: oxidation numbers
There are a select group of elements that rarely, if ever, deviate from a certain oxidation number. Oxygen and Hydrogen are prime examples of this. O will almost always be 2- and H will almost always be 1+. This will give an inkling into the rest of the molecule.
Now, what if these aren't in the molecule, what then? Well, first, try to see if their most preferred oxidation number will suffice. For example, NaCl, where Na is 1+ and Cl is 1-. This is because having these oxidation numbers will give octets to both elements.
If you still can't do this, use the lewis structure to determine the oxidation numbers. Electronegativity is extremely important here. An example can be seen in the picture below. The oxygen atoms in the left molecule that have a 1- oxidation have that because they don't get 1- from the other O they are bonded to, but they do get 1- from the Cr, resulting in a total of 1-. The double bonded O, however, is 2- because it trumps the electronegativity of the Cr.
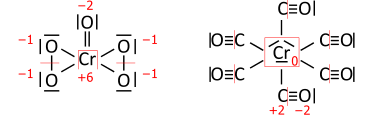
Now, what if these aren't in the molecule, what then? Well, first, try to see if their most preferred oxidation number will suffice. For example, NaCl, where Na is 1+ and Cl is 1-. This is because having these oxidation numbers will give octets to both elements.
If you still can't do this, use the lewis structure to determine the oxidation numbers. Electronegativity is extremely important here. An example can be seen in the picture below. The oxygen atoms in the left molecule that have a 1- oxidation have that because they don't get 1- from the other O they are bonded to, but they do get 1- from the Cr, resulting in a total of 1-. The double bonded O, however, is 2- because it trumps the electronegativity of the Cr.
-
Joshua Eidam 2A
- Posts: 89
- Joined: Wed Sep 30, 2020 9:58 pm
Re: oxidation numbers
Look at a molecule and if there is an element such a oxygen that is always a charge of -2, you can determine the oxidation number based on the charge of the overall molecule. For example, NO2-, the oxidation number of N would be +3 since the two oxygens will add to a charge of -4, and if the whole molecule has a charge of -1, then -4 + 3 = -1.
-
Jennifer Fuentes 2K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:17 am
Re: oxidation numbers
The oxidation number of a monatomic ion equals the charge of the ion. The oxidation number of H is +1, but it is -1 in when combined with less electronegative elements. The oxidation number of O in compounds is usually -2, but it is -1 in peroxides. The oxidation number of a Group 1 element in a compound is +1.
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 6 guests

