1.69 : In a recent suspense film, two secret agents must penetrate a criminal's stronghold monitored by a lithium photomultiplier cell that is continually bathed in light from a laser. If the beam of light is broken, an alarm sounds. The agents want to use a handheld laser to illuminate the cell while they pass in front of it.
They have two lasers, a high intensity red ruby laser (694 nm) and a low intensity violet GaN laser (405 nm), but they disagree on which one would be better.
Determine a) which laser they should use and b) the kinetic energy of the electrons emitted. The work function of lithium 2.93 eV.
1.69 Homework Problem [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Preston_Dang_1B
- Posts: 22
- Joined: Wed Sep 21, 2016 2:59 pm
Re: 1.69 Homework Problem [ENDORSED]
The agents have to choose the laser that best mimics the original laser in regards to it being able to discharge electrons from the surface of the lithium cell. This is because if the beam is broken or if the laser has less energy than the original one, the alarm will go off (indicating that no electrons are being discharged from the lithium cell). You're given the work function of lithium to be 2.93 eV, which is 4.69x10-19 joules. To find the energy of the two lasers, use the 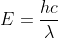 equation. As it turns out, the GaN laser is the best option because it's the only one whose total energy is greater than that of the value of the work function. To find the kinetic energy of the electrons, subtract the work function of lithium from the total energy of the laser.
equation. As it turns out, the GaN laser is the best option because it's the only one whose total energy is greater than that of the value of the work function. To find the kinetic energy of the electrons, subtract the work function of lithium from the total energy of the laser.
-
Chelsey_Schaum1H
- Posts: 15
- Joined: Fri Jul 22, 2016 3:00 am
Re: 1.69 Homework Problem
Hey!
So the work is given in the problem. The work is equivalent to the threshold energy.
First, you need to convert the work from ev to joules
Second, convert each wavelength of light given (the red and violet) into energy using E=hc/(wavelength)
Third, compare the energies you calculated to the threshold energy. The wavelength would have had to have enough energy to surpass the threshold energy in order to be the best light. So, the light with the energy that is greater than the threshold energy is the best choice.
For the kinetic energy
we know the threshold energy, and we know the energy of the incoming photon which is what you calculated in part A
to find Ek you subtract the energy of the incoming photon and the threshold energy
So the work is given in the problem. The work is equivalent to the threshold energy.
First, you need to convert the work from ev to joules
Second, convert each wavelength of light given (the red and violet) into energy using E=hc/(wavelength)
Third, compare the energies you calculated to the threshold energy. The wavelength would have had to have enough energy to surpass the threshold energy in order to be the best light. So, the light with the energy that is greater than the threshold energy is the best choice.
For the kinetic energy
we know the threshold energy, and we know the energy of the incoming photon which is what you calculated in part A
to find Ek you subtract the energy of the incoming photon and the threshold energy
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 7 guests

