Hello,
I was so closeee to getting this problem 100% right.
Can someone please walk me through how to do the last line, (with 2.5 nm given)
Also I did not know how to assign the activity to their respective wavelengths, how do we know which is which (especially since reading and suntan got very close numbers)
here's my work
https://drive.google.com/file/d/0B89MG7gM_cLfZ2FfdE5DZC1ub1k/view
thank you
Ch1 problem9
Moderators: Chem_Mod, Chem_Admin
-
Sarah_Wilen
- Posts: 62
- Joined: Fri Jun 23, 2017 11:39 am
- Been upvoted: 4 times
Re: Ch1 problem9
The last line gave you the wavelength of 2.5 nm. First, convert nanometers to meters by dividing by 10^9 nanometers.
An approach would be to use the speed of light equation to solve for velocity: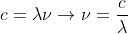
Then, to solve for energy, use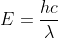 .
.
Another approach would be to solve for energy: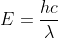 , then solving for frequency from
, then solving for frequency from  and using the energy you just found. I suggest the other method just incase you solve for energy wrong and then everything else is wrong after that.
and using the energy you just found. I suggest the other method just incase you solve for energy wrong and then everything else is wrong after that.
I know that this is how the EMR spectrum goes: radio waves, microwaves, infrared radiation, visible light, ultraviolet, x-ray, gamma-ray
(A tip to memorizing this is listening to this annoyingly catchy song: https://www.youtube.com/watch?v=bjOGNVH3D4Y which is how I've memorized the EMR spectrum since 5th grade)
To figure out the event, I look at the wavelength. I also convert all the wavelengths to nanometers to make the process easier for me. I first look at the shortest and the longest wavelength. The longest wavelength means the lowest energy and the shortest wavelength means the highest energy. The longest is 1 m or 1.0 x 10^9 nm. The event with the least amount of energy is microwave popcorn. The highest energy is the wavelength of 2.5 nm. This event has got to be the dental x-ray. Now, we get into the nitty gritty. I know that suntanning is from UV light which has higher energy than reading. So, I can either remember the range of visible light (400-700 nm) and count the 340 nm wavelength out as visible light or I can compare the energies of the remaining two and see that the higher energy must be suntanning and the lower must be reading.
Hope this helps!
Sent from the really cold produce section of Costco.
An approach would be to use the speed of light equation to solve for velocity:
Then, to solve for energy, use
Another approach would be to solve for energy:
I know that this is how the EMR spectrum goes: radio waves, microwaves, infrared radiation, visible light, ultraviolet, x-ray, gamma-ray
(A tip to memorizing this is listening to this annoyingly catchy song: https://www.youtube.com/watch?v=bjOGNVH3D4Y which is how I've memorized the EMR spectrum since 5th grade)
To figure out the event, I look at the wavelength. I also convert all the wavelengths to nanometers to make the process easier for me. I first look at the shortest and the longest wavelength. The longest wavelength means the lowest energy and the shortest wavelength means the highest energy. The longest is 1 m or 1.0 x 10^9 nm. The event with the least amount of energy is microwave popcorn. The highest energy is the wavelength of 2.5 nm. This event has got to be the dental x-ray. Now, we get into the nitty gritty. I know that suntanning is from UV light which has higher energy than reading. So, I can either remember the range of visible light (400-700 nm) and count the 340 nm wavelength out as visible light or I can compare the energies of the remaining two and see that the higher energy must be suntanning and the lower must be reading.
Hope this helps!
Sent from the really cold produce section of Costco.
Re: Ch1 problem9
LOL the dedication^ but sweet, this clarification really helped me out too. But also, during the exam would it be advisable to convert all our wavelength numbers to nanometers?
-
Sarah_Wilen
- Posts: 62
- Joined: Fri Jun 23, 2017 11:39 am
- Been upvoted: 4 times
Re: Ch1 problem9
Hey, JD,
I personally like to write the answer down in meters, then convert it to nanometers, so it looks like a readable number to me. I think it may depend on the question. If the question asks for the answer in meters, then meters they shall receive. I converted the wavelength into nanometers for this question so that I could compare the numbers easily. I think it's whatever floats your goat. I'm glad you found clarity in my previous post :)
I personally like to write the answer down in meters, then convert it to nanometers, so it looks like a readable number to me. I think it may depend on the question. If the question asks for the answer in meters, then meters they shall receive. I converted the wavelength into nanometers for this question so that I could compare the numbers easily. I think it's whatever floats your goat. I'm glad you found clarity in my previous post :)
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 15 guests

