chapter 1 question 27
Moderators: Chem_Mod, Chem_Admin
-
Cali Rauk1D
- Posts: 51
- Joined: Fri Sep 29, 2017 7:03 am
chapter 1 question 27
I am trying to find how many proton the lamp can generate in 2 seconds. I know I should use the E=hv equation but I am getting stuck at where to use the 32 Jxs^-1.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: chapter 1 question 27
You need to first calculate the amount of energy in Joules the lamp generates in 2 seconds before you can find the number of photons. Calculating the energy of the lamp is simply 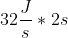 = 64J.
= 64J.
-
Michaela Capps 1l
- Posts: 50
- Joined: Thu Jul 27, 2017 3:00 am
Re: chapter 1 question 27
Okay so you were right to want to use E=h·ν but you rearrange it because v ("nu" or frequency)=c/λ so we can use E=hc/λ =(6.626·10^-34 Js)·( 2.997·10^8m/s)/ (470·10-9 m)=4.225*10^-19 J. The lamp emits 32J/s so in 2 seconds, the lamp emits (32*2=) 64J. So we use E(per mol of photons)=N(photons)*E. This equation was in the book around the Einstein section. So 64J=N(photons)*(4.225*10^-19 J). N(photons)=1.893*10^20 photons. Multiply the number of photons by Avogadro's to get the moles. (1mole/6.022*10^23 photon)=3.144*10^-4 moles
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 9 guests

