Rydberg Formula for Atomic Hydrogen [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Cindy Nguyen 1L
- Posts: 48
- Joined: Fri Apr 06, 2018 11:04 am
Rydberg Formula for Atomic Hydrogen
On the front page of Test 1, the Rydberg Formula is 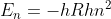 . In lecture, it is
. In lecture, it is 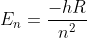 . I was wondering how the formula on Test 1 is equivalent to the one given in lecture.
. I was wondering how the formula on Test 1 is equivalent to the one given in lecture.
-
LilianKhosravi_1H
- Posts: 32
- Joined: Fri Apr 06, 2018 11:03 am
Re: Rydberg Formula for Atomic Hydrogen
The one on my cover of test 1 is -(hR/n^2), which is the same as the one given in lecture.
-
Sarah Brecher 1I
- Posts: 31
- Joined: Fri Apr 06, 2018 11:02 am
Re: Rydberg Formula for Atomic Hydrogen
While the test will give you the E(n) = -(hR)/n^2, a faster equation that I was given in discussion that is very helpful is:
v (frequency) = R [(1/nf^2) - (1/ni^2)]
v (frequency) = R [(1/nf^2) - (1/ni^2)]
-
Endri Dis 1J
- Posts: 33
- Joined: Fri Apr 06, 2018 11:02 am
Re: Rydberg Formula for Atomic Hydrogen
There are actually many forms of Rydberg formula given but the one that I use is
DeltaE = -R*((1/nf^2)-(1/ni^2))*Z^2
Where
DeltaE= diff in energy between the final and initial energy level(J)
R= Rydberg"s compound(2.178X10^-18J)
nf= final energy level
ni= initial energy level
Z=atomic# (# of protons)
Also, Rydberg's constant can be written as
2.178X10^-18J
3.29X10^15s^-1
1.097X10^7m^-1
DeltaE = -R*((1/nf^2)-(1/ni^2))*Z^2
Where
DeltaE= diff in energy between the final and initial energy level(J)
R= Rydberg"s compound(2.178X10^-18J)
nf= final energy level
ni= initial energy level
Z=atomic# (# of protons)
Also, Rydberg's constant can be written as
2.178X10^-18J
3.29X10^15s^-1
1.097X10^7m^-1
-
Erin Li 1K
- Posts: 30
- Joined: Fri Apr 06, 2018 11:03 am
Re: Rydberg Formula for Atomic Hydrogen [ENDORSED]
it's E(n) = -(hR)/nfinal^2 -(hR)/ninitial^2
and for frequency it is = R [(1/nfinal^2) - (1/ninitial^2)]
and for frequency it is = R [(1/nfinal^2) - (1/ninitial^2)]
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 6 guests

