Calculating amount of photons
Moderators: Chem_Mod, Chem_Admin
-
Phoebe Chen 4I
- Posts: 55
- Joined: Fri Sep 28, 2018 12:29 am
Calculating amount of photons
Does anyone know how to calculate the second part of the question? How many photons of infrared radiation does the lamp generate in 1.0s? Is there a mathematical equation?
-
Ashley Zhu 1A
- Posts: 69
- Joined: Fri Sep 28, 2018 12:16 am
Re: Calculating amount of photons
After finding the energy of a photon using the equation, E = hf (f = frequency), you just need to divide the total energy emitted (11J) by the energy per photon to get the number of photons: 11J/(1.074 x 10^-19 J).
-
505194972 3k
- Posts: 27
- Joined: Fri Sep 28, 2018 12:25 am
Re: Calculating amount of photons
Ashley Zhu 1E wrote:After finding the energy of a photon using the equation, E = hf (f = frequency), you just need to divide the total energy emitted (11J) by the energy per photon to get the number of photons: 11J/(1.074 x 10^-19 J).
How did you find that value for energy per photon?
-
Ashley Zhu 1A
- Posts: 69
- Joined: Fri Sep 28, 2018 12:16 am
Re: Calculating amount of photons
The energy per photon can be found using the equation E = hv where h is Planck's constant and v is the frequency, which you can find from the given wavelength with the [speed of light = wavelength x frequency] equation.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Calculating amount of photons
You can use the following equation to find the amount of energy per photon:
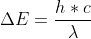
Plug in the values, but you would need to convert 1850nm to m.
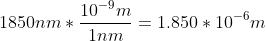
Planck's constant can be substituted for h
(3.00*10^{8}m/s)}{1.850*10^{-6}m})
With units cancelled, E would then equal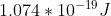 per photon. Because you know the total amount of Joules emitted (11J), you could then divide 11J/1.074*10^-19 J to find the total number of photons.
per photon. Because you know the total amount of Joules emitted (11J), you could then divide 11J/1.074*10^-19 J to find the total number of photons.
Plug in the values, but you would need to convert 1850nm to m.
Planck's constant can be substituted for h
With units cancelled, E would then equal
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 11 guests

