In the ultraviolet spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emission of energy that leads to this spectral line.
I was looking at the solution manual and had trouble interpreting how to get n2. Can someone further explain how they conceptualized this problem and how to solve it?
1A. 15
Moderators: Chem_Mod, Chem_Admin
-
Ellen Amico 2L
- Posts: 101
- Joined: Thu Sep 19, 2019 12:16 am
Re: 1A. 15
I started off by finding the frequency using the wavelength and then I used the equation: frequency = -R[1/(n1)^2- 1/(n2)^2] and solved for n1, which should get you a number close to 3. I figured out n2 because the question mentioned UV radiation, which equates to the Lyman series and n2 is always 1 in that series.
-
Ruby Tang 2J
- Posts: 102
- Joined: Fri Aug 30, 2019 12:15 am
Re: 1A. 15
1), First, you need to know that the ultraviolet spectrum of atomic hydrogen corresponds to the Lyman series (defined as a transition between n=1 and n>=2). For this class, I would remember this in addition to the fact that the visible spectrum corresponds to the Balmer series (transition between n=2 and n>=3).
2) Then, in order to get to the shortcut used in the solution manual, we can take the Rydberg equation E=-hR/n^2 and substitute E for hv. Now we have hv=-hR/n^2 and we can cancel out the h's on both sides to get v=-R/n^2.
3) In order to find v, we can use v=c/λ (you should get ~2.924*10^15 Hz)
4) Now in order to find the change in energy, we can use E=Ef - Ei. We can now use the v=-R/n^2 equation from step 2. You should get v=-R/n2^2 - (-R/n1^2), which is the same thing as v=R/n1^2 - R/n2^2, or v=R(1/n1^2-1/n2^2). You have the frequency (v), the Rydberg constant (R), and n1 (which is by definition 1), so now n2 is the only unknown variable and you should be able to solve for it.
2) Then, in order to get to the shortcut used in the solution manual, we can take the Rydberg equation E=-hR/n^2 and substitute E for hv. Now we have hv=-hR/n^2 and we can cancel out the h's on both sides to get v=-R/n^2.
3) In order to find v, we can use v=c/λ (you should get ~2.924*10^15 Hz)
4) Now in order to find the change in energy, we can use E=Ef - Ei. We can now use the v=-R/n^2 equation from step 2. You should get v=-R/n2^2 - (-R/n1^2), which is the same thing as v=R/n1^2 - R/n2^2, or v=R(1/n1^2-1/n2^2). You have the frequency (v), the Rydberg constant (R), and n1 (which is by definition 1), so now n2 is the only unknown variable and you should be able to solve for it.
-
Sebastian Lee 1L
- Posts: 157
- Joined: Fri Aug 09, 2019 12:15 am
- Been upvoted: 1 time
Re: 1A. 15
Okay so we know that the energy of the light emitted is caused by the transition of the electron from one energy level to another. We can represent this as 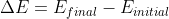 . We also know that, for hydrogen, the energy of a specific energy level is given by the equation
. We also know that, for hydrogen, the energy of a specific energy level is given by the equation 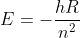 . We also know the equations:
. We also know the equations: 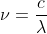 and
and 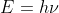 . From here you would solve for the frequency of light emitted from the wavelength, and then the energy of that light. This is delta E (note: that since energy is emitted delta E should be negative). You also know that the final energy level is level 1 because the light emitted is ultraviolet (Lyman series). You can solve for the final energy of n=1 and then use algebra to solve for the initial energy level.
. From here you would solve for the frequency of light emitted from the wavelength, and then the energy of that light. This is delta E (note: that since energy is emitted delta E should be negative). You also know that the final energy level is level 1 because the light emitted is ultraviolet (Lyman series). You can solve for the final energy of n=1 and then use algebra to solve for the initial energy level.
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 9 guests

