In each second, a certain lamp produces 2.4 x 10^21 photons
with a wavelength of 633 nm. How much power (in watts) is produced as radiation at this wavelength (1W=1 J/s)?
What is the answer and how do we solve this problem?
Focus 1.3
Moderators: Chem_Mod, Chem_Admin
-
Julie_Reyes1B
- Posts: 105
- Joined: Sat Jul 20, 2019 12:16 am
Re: Focus 1.3
Hi,
The answer in the text book is 750 W. First, use the equation c=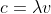 to solve for frequency. Then, use the equation E=hv to solve for the energy of one photon. Then, multiply your answer by the number of photons indicated in the problem. Since this is the amount of energy released each second, the answer is in Joules per seconds, which is Watts.
to solve for frequency. Then, use the equation E=hv to solve for the energy of one photon. Then, multiply your answer by the number of photons indicated in the problem. Since this is the amount of energy released each second, the answer is in Joules per seconds, which is Watts.
Hope this helps!
The answer in the text book is 750 W. First, use the equation c=
Hope this helps!
-
Cynthia Rodas 4H
- Posts: 51
- Joined: Wed Sep 18, 2019 12:21 am
Re: Focus 1.3
In order to find the amount of power produced in watts, you would use the equation:
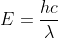
This equation is used to help you find the energy per photon. From here, you would plug in Planck's constant (6.62608 x 10-34 J.s), the speed of light (2.998 x 108 m/s), and the given wavelength converted into meters (633 x 10 -9 m).
After plugging in everything, you should get 3.14 x 10^-19 J/photon. So then you would multiply that by 2.4 x 10^21 photons to get 750 J. Recall that watts is 1 J/s, so you would divide the answer by 1 second to get 750 J/s.
This equation is used to help you find the energy per photon. From here, you would plug in Planck's constant (6.62608 x 10-34 J.s), the speed of light (2.998 x 108 m/s), and the given wavelength converted into meters (633 x 10 -9 m).
After plugging in everything, you should get 3.14 x 10^-19 J/photon. So then you would multiply that by 2.4 x 10^21 photons to get 750 J. Recall that watts is 1 J/s, so you would divide the answer by 1 second to get 750 J/s.
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 11 guests

