Q.15:
"You use an electron microscope in which the matter wave associated with the electron beam has a wavelength of 0.0449
nm. What is the kinetic energy of an electron in the beam, expressed in electron volts"
Very confused on homework question!
Moderators: Chem_Mod, Chem_Admin
-
Yuzhe Yuan
- Posts: 104
- Joined: Fri Sep 24, 2021 6:38 am
- Been upvoted: 1 time
Re: Very confused on homework question!
Hi,
First use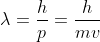
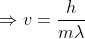 to calculate the speed of the electron;
to calculate the speed of the electron;
Then, use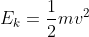 to calculate the kinetic energy of the electron;
to calculate the kinetic energy of the electron;
Finally, convert J to eV.
First use
Then, use
Finally, convert J to eV.
-
Sristi Palimar 2E
- Posts: 105
- Joined: Fri Sep 24, 2021 5:47 am
- Been upvoted: 2 times
Re: Very confused on homework question!
You can solve for kinetic energy by using the equation 1/2*mass*velocity^2. You already know the mass of the electron (see constants and equations sheet), therefore you only need to solve for velocity. You can use the de Broglie wavelength formula to calculate the velocity. Recall that the de Broglie wavelength formula is lambda = h/p, where p=mass(*velocity). The problem gives you the wavelength of the electron (remember to convert to meters), you know planks constant (see constants and equations sheet), and you know the mass of the electron.
Once you've found the kinetic energy, you can convert joules to electronic volts by multiplying your answer by 6.242*(10)^18
Once you've found the kinetic energy, you can convert joules to electronic volts by multiplying your answer by 6.242*(10)^18
-
Jiane_Beach_1D
- Posts: 52
- Joined: Fri Sep 24, 2021 6:07 am
Re: Very confused on homework question!
The question is saying that you have an electron beam with a wavelength of 0.0449. This means that the wavelength of the electrons is 0.0449. If you know the wavelength then you can use the equation lambda = h/mv and solve for v. Because you are looking for kinetic energy, you need to use the equation E = 1/2 mv^2. Using the velocity you found in the first equation, you can now solve for E. This energy number will be in J, so you need to convert it to eV.
-
Diana peng 3I
- Posts: 31
- Joined: Thu Sep 30, 2021 5:05 am
Re: Very confused on homework question!
Yuzhe Yuan wrote:Hi,
First use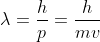
to calculate the speed of the electron;
Then, useto calculate the kinetic energy of the electron;
Finally, convert J to eV.
This post help me a lot, I keeps getting it wrong. Turn out I forgot to convert
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 4 guests

