14.15 (b) states: Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions:
(b) H+(aq) + OH-(aq) ---> H2O(l), the Brønsted neutralization reaction
I am looking at the solutions manual and I am really confused. Can someone explain in detail how to get to the final answer (including how to get the half-reactions)?
14.15 (b)
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: 14.15 (b)
We're looking for half reactions involving H2O, H+, and OH-. A good option could be using the reactions suggested in the solutions manual, but this isn't our only choice. Just going by the solution manual, they started with the reduction on O2 gas in acidic conditions (our H+). Logically, we need to cancel out the O2 gas in the oxidation reaction because it doesn't appear in our desired reaction. Another reaction involving O2 gas is the oxidation of OH-, perfect for what we need. Since that will be our oxidation half reaction, we need to flip the sign of the E0 i.e. -0.40 V, so E0cell will be 1.23 V + (-0.40 V) = 0.83 V
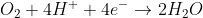
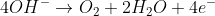
Notice that this same result could have been obtained by the reduction of H+, E0 0.0 V, and the oxidation of hydrogen gas to water, E0red = -0.83 V and E0cell = 0 + 0.83 V = 0.83 V
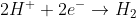
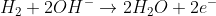
Notice that this same result could have been obtained by the reduction of H+, E0 0.0 V, and the oxidation of hydrogen gas to water, E0red = -0.83 V and E0cell = 0 + 0.83 V = 0.83 V
-
sandyh9711
- Posts: 21
- Joined: Fri Sep 25, 2015 3:00 am
Return to “Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams”
Who is online
Users browsing this forum: No registered users and 10 guests

