Test 2, Question #7 [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Hena Sihota 1L
- Posts: 51
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 2 times
Test 2, Question #7
What is the correct answer for this question: Calculate the standard potential for the following reaction using the standard reduction potentials on the last page of the test: Cr2O72-(aq)+14H+(aq)+12e-->2Cr(s)+7H2O(l)
-
Cam Bear 2F
- Posts: 60
- Joined: Thu Jul 27, 2017 3:01 am
Re: Test 2, Question #7
This is similar to HW problem 14.27. So basically to get Cr2O72-(aq)+14H+(aq)+12e-->2Cr(s)+7H2O(l) from the reactions given on the last page of the test you have to combine the two reactions Cr2O72-(aq)+14H+(aq)+6e-->2Cr3+(aq)+7H2O(l) and Cr3+(aq)+3e--->Cr(s).
However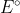 isn't a state function so you can't just simply add up both of the
isn't a state function so you can't just simply add up both of the 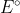 .
.
Instead, because Gibbs free energy is a state function, you convert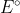 of each half reaction to change in Gibbs free energy using
of each half reaction to change in Gibbs free energy using 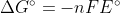 .
.
First you have to multiply the second half reaction by 2 to get the right final reaction. Then you plug everything into the equation.
So of the final reaction=
of the final reaction=
of the first reaction + 2 times of the second reaction.
of the second reaction.
However
Instead, because Gibbs free energy is a state function, you convert
First you have to multiply the second half reaction by 2 to get the right final reaction. Then you plug everything into the equation.
So
of the first reaction + 2 times
-
Anh Nguyen 2A
- Posts: 36
- Joined: Fri Sep 29, 2017 7:05 am
- Been upvoted: 1 time
Re: Test 2, Question #7 [ENDORSED]
Cr2O72-(aq) +14H+(aq) +12e- -> 2Cr(s) +7H2O(l)
This reaction is made up of 2 reactions:
Cr2O72-(aq) +14H+(aq) +6e- -> 2Cr3+(aq) + 7H2O(l) E1=+1.33V
2Cr3+(aq) +6e- -> 2Cr(s) E2=-0.74V
ΔG=ΔG1+ΔG2
<=> -nFE = -n1FE1 - n2FE2
<=> -12FE = -6FE1 -6FE2
<=> E = 1/2E1 + 1/2E2
<=> E= 1/2x(1.33) + 1/2x-0.74 = 0.30V
This reaction is made up of 2 reactions:
Cr2O72-(aq) +14H+(aq) +6e- -> 2Cr3+(aq) + 7H2O(l) E1=+1.33V
2Cr3+(aq) +6e- -> 2Cr(s) E2=-0.74V
ΔG=ΔG1+ΔG2
<=> -nFE = -n1FE1 - n2FE2
<=> -12FE = -6FE1 -6FE2
<=> E = 1/2E1 + 1/2E2
<=> E= 1/2x(1.33) + 1/2x-0.74 = 0.30V
Return to “Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams”
Who is online
Users browsing this forum: No registered users and 16 guests

