galvanic vs electrolytic
Moderators: Chem_Mod, Chem_Admin
-
Jacob Puchalski 1G
- Posts: 97
- Joined: Fri Aug 09, 2019 12:16 am
-
JonathanS 1H
- Posts: 101
- Joined: Thu Jul 11, 2019 12:17 am
Re: galvanic vs electrolytic
A galvanic cell has a positive Eocell value, and therefore the redox reactions and flow of e- is spontaneous. An electrolytic cell has a negative Eocell value, so the flow of electrons requires an external source of current to cause the redox reactions to occur.
-
Isabella Dal Porto 1H
- Posts: 100
- Joined: Fri Aug 30, 2019 12:16 am
Re: galvanic vs electrolytic
For galvanic cells, spontaneous redox reactions convert the chemical energy to an electric energy. For electrolytic cells, non-spontaneous redox reactions convert the electric energy to a chemical energy.
-
Shivam Rana 1D
- Posts: 106
- Joined: Fri Aug 09, 2019 12:16 am
Re: galvanic vs electrolytic
A galvanic cell prodices electrical energy and an electrolytic cell produces chemical energy.
-
TimVintsDis4L
- Posts: 104
- Joined: Sat Aug 17, 2019 12:17 am
Re: galvanic vs electrolytic
Galvanic Cell and Electrolytic Cells are essentially opposites
Galvanic Cell =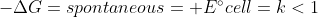
Electrolytic Cell =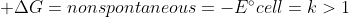
Galvanic Cell =
Electrolytic Cell =
Re: galvanic vs electrolytic
Galvanic cells use spontaneous reactions. Electrolytic cells are non-spontaneous, meaning that energy is needed to force the reaction to happen.
-
RasikaObla_4I
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
Re: galvanic vs electrolytic
A galvanic cell have a positive standard Ecell while an electrolytic cell have a negative standard Ecell and it is not spontaneous.
Re: galvanic vs electrolytic
In a electrolytic cell, energy from an external power source is used to drive a normally non-spontaneous reaction. In a galvanic cell, chemical energy is converted into electrical energy. The redox reaction is spontaneous and the anode is negative and the cathode is the positive electrode.
-
Jeremy_Guiman2E
- Posts: 82
- Joined: Fri Sep 28, 2018 12:29 am
Re: galvanic vs electrolytic
Galvanic cells transforms energy from spontaneous redox reactions into electrical energy. Electrolytic cells convert electrical energy into chemical energy.
-
J Medina 2I
- Posts: 102
- Joined: Wed Sep 25, 2019 12:17 am
Re: galvanic vs electrolytic
When Galvanic cells are brought up it helps me to immediately think of batteries to help me remember that Galvanic cells convert chemical energy into electrical energy.
-
Jarrett Peyrefitte 2K
- Posts: 102
- Joined: Sat Aug 24, 2019 12:16 am
Re: galvanic vs electrolytic
Electrolytic cells - non-spontaneous, convert electric energy into chemical energy
Galvanic cells - spontaneous, convert chemical energy into electric energy
Galvanic cells - spontaneous, convert chemical energy into electric energy
Last edited by Jarrett Peyrefitte 2K on Mon Mar 09, 2020 12:04 am, edited 1 time in total.
-
Nawal Dandachi 1G
- Posts: 102
- Joined: Sat Sep 28, 2019 12:16 am
Re: galvanic vs electrolytic
galvanic cells have a positive standard E and are spontaneous while electrolytic cells have a negative standard E and are nonspontaneous.
-
Eesha Chattopadhyay 2K
- Posts: 104
- Joined: Fri Aug 09, 2019 12:16 am
Re: galvanic vs electrolytic
Galvanic cells are cells that use spontaneous reactions. Electrolytic cells use non-spontaneous reactions.
Return to “Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams”
Who is online
Users browsing this forum: No registered users and 7 guests

