Finding standard potential
Moderators: Chem_Mod, Chem_Admin
-
Vincent Cao 2D
- Posts: 36
- Joined: Mon Jan 09, 2023 9:00 am
Finding standard potential
I know that inside of a galvanic cell, we always have to do E knot right - E knot left, however, somewhere I saw that we had to convert the E knot into their respective delta G, then add the delta G together, then change it back into E knot. In which scenarios do you do which?
-
zjfoster25
- Posts: 34
- Joined: Mon Jan 09, 2023 2:29 am
Re: Finding standard potential
Hey Vincent, just found this in the book. Essentially, you need to do this when the standard potential for a half reaction is not given by a table we are using. The example they use in the book is when you are trying to find the standard potential of the half reaction 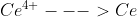 but we only have the potentials for
but we only have the potentials for 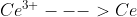 and
and 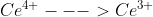 . The textbook goes into further detail in example 6M.2.
. The textbook goes into further detail in example 6M.2.
-
Cassidy Sadowski 3E
- Posts: 38
- Joined: Mon Jan 09, 2023 10:01 am
Re: Finding standard potential
E cathode - E anode can only be used in the case of a galvantic cell or a reduction-oxidation. In the example I believe you're referring to they were both either a reduction or oxidation (I can't remember) but in that case we are unable to combine the E values to reach the answer. We have to go through delta G.
Hope that makes sense!
Hope that makes sense!
-
samaagwani-disc2L
- Posts: 34
- Joined: Mon Jan 09, 2023 9:38 am
Re: Finding standard potential
Hello, in this case, we cannot combine the E values and we have to use the delta Gs. Remember that the E value equation can only be used with a galvanic cell or a redox reaction. The example that somebody else provided is definitely worth taking a look at! I hope this helps:)
Return to “Work, Gibbs Free Energy, Cell (Redox) Potentials”
Who is online
Users browsing this forum: No registered users and 2 guests

