swapping signs of E values
Moderators: Chem_Mod, Chem_Admin
-
Miranda 1J
- Posts: 51
- Joined: Fri Sep 29, 2017 7:06 am
swapping signs of E values
When we are trying to find the Ecell how do we know which one of the two equations we swap the sign of?
Re: swapping signs of E values
If you use the formula 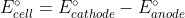 you do not need to change the sign of
you do not need to change the sign of 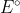 for either half reaction because the minus sign does it for you.
for either half reaction because the minus sign does it for you.
-
Diego Zavala 2I
- Posts: 65
- Joined: Fri Sep 29, 2017 7:07 am
- Been upvoted: 1 time
-
Rachel Formaker 1E
- Posts: 86
- Joined: Fri Sep 29, 2017 7:04 am
- Been upvoted: 2 times
Re: swapping signs of E values
I think Dr. Lavelle prefers that we use:
Eocell = Eocathode - Eoanode
so that we don't have to switch the sign of either standard reduction potential
Eocell = Eocathode - Eoanode
so that we don't have to switch the sign of either standard reduction potential
-
sahiltelang-Discussion 1J
- Posts: 50
- Joined: Thu Jul 13, 2017 3:00 am
Re: swapping signs of E values
The main thing you have to remember when you use the Cathode-Anode equation is that both the Enaught values that you use have to be reduction half reaction potential values.
-
Jennie Fox 1D
- Posts: 66
- Joined: Sat Jul 22, 2017 3:01 am
Re: swapping signs of E values
I use Eo(cell) = Eo(cathode) - Eo(anode) so that way I do not need to flip any signs.
-
Ridhi Ravichandran 1E
- Posts: 35
- Joined: Sat Jul 22, 2017 3:01 am
Re: swapping signs of E values
You usually swap the sign of the equation that needs to be oxidized. Since the back of the book only lists reduction potentials, and a redox reaction has both reduction and oxidation half reactions, we need to flip one of the equations to turn it into an oxidation. Turning a half reaction from a reduction to an oxidation basically means you are reversing the equation, in which case you need to change the sign of E naught.
-
Shane Simon 2K
- Posts: 30
- Joined: Fri Sep 29, 2017 7:05 am
Re: swapping signs of E values
Yeah I also do what Ridhi does which is flipping the sign of the oxidation reaction since the appendix contains the reduction potentials. Either way works though, it all just depends on what way works best for you.
-
Abby Ellstrom 1I
- Posts: 53
- Joined: Fri Sep 29, 2017 7:04 am
Re: swapping signs of E values
You swipe the sign on the anode by using the equation Ecell=Ecathode-Eanode
Return to “Work, Gibbs Free Energy, Cell (Redox) Potentials”
Who is online
Users browsing this forum: No registered users and 2 guests

