negative sign
Moderators: Chem_Mod, Chem_Admin
-
Sophia Dinh 1D
- Posts: 100
- Joined: Thu Jul 25, 2019 12:15 am
-
Jessa Maheras 4F
- Posts: 121
- Joined: Fri Aug 02, 2019 12:16 am
-
Indy Bui 1l
- Posts: 99
- Joined: Sat Sep 07, 2019 12:19 am
Re: negative sign
I believe it has to do with the relationship between E and delta G. When E is positive, delta G should be negative meaning it is spontaneous.
Re: negative sign
Energy = -work/Charge;
Rearrange this to get work = -E*Charge
From the Farraday's constant, Charge = n*F
Therefore work = -n*F*E
At constant temp and pressure, work max = deltaG
Therefore deltaG = -n*F*E
Rearrange this to get work = -E*Charge
From the Farraday's constant, Charge = n*F
Therefore work = -n*F*E
At constant temp and pressure, work max = deltaG
Therefore deltaG = -n*F*E
-
Ashley Alvarado 2C
- Posts: 45
- Joined: Sat Aug 17, 2019 12:16 am
Re: negative sign
delta G*= -nFE
w(max)=-nFE
The system is doing work, so energy leaves the system resulting in -w.
w(max)=-nFE
The system is doing work, so energy leaves the system resulting in -w.
-
Jared_Yuge
- Posts: 100
- Joined: Sat Aug 17, 2019 12:17 am
-
Frankie Mele 3J
- Posts: 104
- Joined: Wed Sep 30, 2020 10:09 pm
Re: negative sign
Favorable redox reactions have positive voltage differences which results in E being a positive value and deltaG therefore being negative.
-
Lesly Lopez 3A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:54 pm
- Been upvoted: 1 time
Re: negative sign
The negative sign is important because it is telling you that the reaction has released or lost energy. The relationship between E and 
G is that when E is positive, G should be negative meaning the reaction is spontaneous.
G should be negative meaning the reaction is spontaneous.
G is that when E is positive,
-
Samantha Lee 1A
- Posts: 108
- Joined: Wed Sep 30, 2020 10:05 pm
- Been upvoted: 1 time
Re: negative sign
Yes! There is a negative sign in front of the n. The equation is 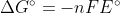
When the reaction is spontaneous, the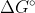 is negative and
is negative and 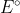 is positive. In order for that to occur, there must be a negative sign in the equation.
is positive. In order for that to occur, there must be a negative sign in the equation.
When the reaction is spontaneous, the
-
Kimiya Aframian IB
- Posts: 131
- Joined: Wed Sep 30, 2020 9:34 pm
Re: negative sign
Sophia Dinh 1D wrote:why is there a negative sign in front of n in the standard Gibbs energy equation?
Hi! I think the negative sign in front of the n (mole value) is because it keeps the relationship between E, delta G, and spontaneity consistent. By this I mean that we need the E value to be positive when delta G is negative. Hope this helps!
-
Dominic Benna 2E
- Posts: 103
- Joined: Wed Sep 30, 2020 10:09 pm
Re: negative sign
A spontaneous reaction has a positive delta E, yet a negative delta G. So, in order to relate the two to each other, the negative sign is added.
-
Edison Tham 3D
- Posts: 50
- Joined: Mon Jun 17, 2019 7:25 am
- Been upvoted: 1 time
Re: negative sign
The system is doing work and based on the fact that ∆G = maximum work, this would mean that there would be a negative sign.
Return to “Work, Gibbs Free Energy, Cell (Redox) Potentials”
Who is online
Users browsing this forum: No registered users and 8 guests

