E cell, K and Delta G
Moderators: Chem_Mod, Chem_Admin
-
Lindsey 3C
- Posts: 46
- Joined: Mon Jan 09, 2023 9:51 am
E cell, K and Delta G
Can someone conceptually explain Achieve #14 on the relationship between E cell, K, and Delta G?
-
Brianna Bui 2J
- Posts: 37
- Joined: Mon Jan 09, 2023 9:28 am
Re: E cell, K and Delta G
Hi!
The equations I find helpful in thinking through these relationships are
deltaG° = -RTIn (K)
deltaG° = -nFE°cell
E°cell = (deltaG°)/nF
when K=1 (system is at equilibrium), delta G° and E°cell are equal to zero.
For delta G, it is zero because K=1 and ln(K) = ln1=0. Since the lnK term is multiplied to the others in the delta G° equation, delta G° is 0.
For E°cell, if delta G° is 0, then the whole numerator is 0, thus the whole equation equals zero.
So, to consider the relationship between E cell, K, and Delta G, I use these equations and pay special attention to how changing the value of K (which can be done by plugging in a number of your choice)
affects delta G, which will then affect E Cell. Hope this helped!
The equations I find helpful in thinking through these relationships are
deltaG° = -RTIn (K)
deltaG° = -nFE°cell
E°cell = (deltaG°)/nF
when K=1 (system is at equilibrium), delta G° and E°cell are equal to zero.
For delta G, it is zero because K=1 and ln(K) = ln1=0. Since the lnK term is multiplied to the others in the delta G° equation, delta G° is 0.
For E°cell, if delta G° is 0, then the whole numerator is 0, thus the whole equation equals zero.
So, to consider the relationship between E cell, K, and Delta G, I use these equations and pay special attention to how changing the value of K (which can be done by plugging in a number of your choice)
affects delta G, which will then affect E Cell. Hope this helped!
-
Mei Tsao 1A
- Posts: 34
- Joined: Mon Jan 09, 2023 2:19 am
Re: E cell, K and Delta G
The solution explains how we use three equations to determine the relationship between E cell, K, and Delta G:
)
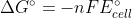
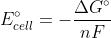
By plugging in the given K values we can determine how the values of E cell and delta G are affected:
When K=1, they plugged it into the first G equation (since given K) and the E cell equation, and the equations show that when K=1, delta G and E cell both = 0.
When K<1, the logarithm is negative which means ln(K) is also negative.
When K>1, the logarithm is positive which means ln(K) is also positive.
Combining the first two equations:=nFE^{\circ}_{cell})
we can see that the sign of ln(K) will affect the entire equation (so if ln(K) is negative E cell is also negative; if ln(K) is positive E cell is also positive).
Using the first equation to find the delta G relationship, we can see that since it contains a negative, whatever sign ln(K) is, delta G will have the opposite sign (if ln(K) is negative, then end result is positive; if ln(K) positive, then end result is negative because of the negative sign).
TLDR: When K=1, everything = 0 when you plug it into the equations.
When K<1, everything else will also be <0 except for delta G (it will be >0, because the negative sign in the delta G equation flips it).
When K>1, everything else will also be >1 except for delta G (it will be <0, because of the negative sign again). Sorry for length of post!
By plugging in the given K values we can determine how the values of E cell and delta G are affected:
When K=1, they plugged it into the first G equation (since given K) and the E cell equation, and the equations show that when K=1, delta G and E cell both = 0.
When K<1, the logarithm is negative which means ln(K) is also negative.
When K>1, the logarithm is positive which means ln(K) is also positive.
Combining the first two equations:
we can see that the sign of ln(K) will affect the entire equation (so if ln(K) is negative E cell is also negative; if ln(K) is positive E cell is also positive).
Using the first equation to find the delta G relationship, we can see that since it contains a negative, whatever sign ln(K) is, delta G will have the opposite sign (if ln(K) is negative, then end result is positive; if ln(K) positive, then end result is negative because of the negative sign).
TLDR: When K=1, everything = 0 when you plug it into the equations.
When K<1, everything else will also be <0 except for delta G (it will be >0, because the negative sign in the delta G equation flips it).
When K>1, everything else will also be >1 except for delta G (it will be <0, because of the negative sign again). Sorry for length of post!
-
Nick Kraemer 2J
- Posts: 34
- Joined: Mon Jan 09, 2023 9:27 am
Re: E cell, K and Delta G
Hi!
Given a K, you can predict the sign of Eo cell because of the equation Eo = (RT)/(nF) x lnK. When K is less than one, the Eo cell is negative because the lnK would be negative. Same for delta Go, but with the equation -RTlnK. Then, repeat the process for the other values of K. Each term is completely dependent on the sign of lnK.
Hope this helps!
Given a K, you can predict the sign of Eo cell because of the equation Eo = (RT)/(nF) x lnK. When K is less than one, the Eo cell is negative because the lnK would be negative. Same for delta Go, but with the equation -RTlnK. Then, repeat the process for the other values of K. Each term is completely dependent on the sign of lnK.
Hope this helps!
Re: E cell, K and Delta G
There are 3 equations you should know to solve this problem:
Delta G° = -RTInK
Dealt G° = -nFE knot cell
E knot cell = (Dealta G°)/nF
If K=1 in the first equation, the delta G and E knot cell are equal to 0 as ln 1 is 0.
So in the last equation, if delta G is 0 the numerator is 0 making it also equal to 0.
Hope this helps!
Delta G° = -RTInK
Dealt G° = -nFE knot cell
E knot cell = (Dealta G°)/nF
If K=1 in the first equation, the delta G and E knot cell are equal to 0 as ln 1 is 0.
So in the last equation, if delta G is 0 the numerator is 0 making it also equal to 0.
Hope this helps!
-
samaagwani-disc2L
- Posts: 34
- Joined: Mon Jan 09, 2023 9:38 am
Re: E cell, K and Delta G
Hello, you should remember that there are three equations needed to solve this problem. I have written them below:
delta G = -RTlnK
Dealt G° = -nFE knot cell
E knot cell = (Dealta G°)/nF
when K=1 the system is in equilibrium and delta G° and E°cell are equal to zero. For E°cell, if delta G° is 0, then the whole numerator is 0, and the whole equation equals zero. Hope this helps:)
delta G = -RTlnK
Dealt G° = -nFE knot cell
E knot cell = (Dealta G°)/nF
when K=1 the system is in equilibrium and delta G° and E°cell are equal to zero. For E°cell, if delta G° is 0, then the whole numerator is 0, and the whole equation equals zero. Hope this helps:)
Who is online
Users browsing this forum: No registered users and 1 guest

