On Question 8B on the 2016 Midterm, the answer says the potential of the cell would increase, because Cadmium ions are taken out of the solution, and the equilibrium will favor the products, driving the reaction forward.
Why does the cell potential increase when the reaction is driven forward?
Midterm 2016 Question 8B [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Midterm 2016 Question 8B [ENDORSED]
Cell potential comes from the differing reaction rates forwards and backwards, so there is a difference in charge, thus, when the reaction shifts more to one side, there is greater potential, so when it shifts to products, the reaction has a greater difference in charge, increasing the cell potential.
-
Isabel Gutierrez 2G
- Posts: 12
- Joined: Wed Sep 21, 2016 2:58 pm
Re: Midterm 2016 Question 8B
I understand that the cell potential correlates to which side the reaction is shifting toward, however I am confused as to why Cd2+ is removed?
-
Maggie Bui 1H
- Posts: 35
- Joined: Fri Jul 22, 2016 3:00 am
- Been upvoted: 1 time
Re: Midterm 2016 Question 8B
Cadmium ions are removed because they react with the sulfide ions that dissociate from sodium sulfide, and precipitate as CdS.
Re: Midterm 2016 Question 8B
Could you also justify this mathematically with 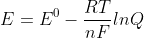 by saying that Q gets smaller and therefore E is larger?
by saying that Q gets smaller and therefore E is larger?
-
Joseph Nguyen 3L
- Posts: 24
- Joined: Fri Jul 22, 2016 3:00 am
Re: Midterm 2016 Question 8B
504829531 wrote:Could you also justify this mathematically withby saying that Q gets smaller and therefore E is larger?
Yes, you can. Cell potential is dependent on ion concentration, the number of moles, and the temperature of the reaction, and you can see this through the Nernst Equation. If Q is smaller, E must be larger because you are subtracting a smaller number. If T is more, then E is smaller. If the number of moles (n) increases, then that term is smaller so the cell potential would increase.
Who is online
Users browsing this forum: No registered users and 10 guests

