Nernst
Moderators: Chem_Mod, Chem_Admin
-
Hui Qiao Wu 1I
- Posts: 56
- Joined: Fri Aug 30, 2019 12:16 am
Re: Nernst
E = Eo -(RT/nF) ln Q
There are also other equations you can derive from this equation that can be considered as the Nernst equation.
There are also other equations you can derive from this equation that can be considered as the Nernst equation.
-
Sanjana K - 2F
- Posts: 102
- Joined: Sat Sep 07, 2019 12:17 am
- Been upvoted: 1 time
Re: Nernst
It's an equation that helps you find the cell potential under non-standard conditions & relates the Q value to the cell potential.
-
Miriam Villarreal 1J
- Posts: 105
- Joined: Sat Aug 17, 2019 12:16 am
Re: Nernst
Nernst equations is used to find Ecell under non-standard conditions. when you are given the Ecell°potential you subtarct it from RT/nF and multiply times ln Q
-
TimVintsDis4L
- Posts: 104
- Joined: Sat Aug 17, 2019 12:17 am
Re: Nernst
Instead of having to plug in all of those constants you can remember it as something that looks like this
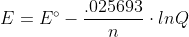
Here the RT/F is converted into .025693 to make the equation simpler.
Here the RT/F is converted into .025693 to make the equation simpler.
-
Manav Govil 1B
- Posts: 104
- Joined: Sat Sep 07, 2019 12:19 am
Re: Nernst
If you are struggling with the Nernst equation, I suggest watching this video:
https://www.khanacademy.org/science/che ... t-equation
It's pretty helpful!
https://www.khanacademy.org/science/che ... t-equation
It's pretty helpful!
-
Mulin_Li_2J
- Posts: 105
- Joined: Sat Aug 17, 2019 12:16 am
Re: Nernst
Ecell = E*cell - (RT/nF)lnQ.
The equation reveals the relationship between concentrations and Ecell.
The equation reveals the relationship between concentrations and Ecell.
-
Jesse H 2L
- Posts: 58
- Joined: Fri Aug 09, 2019 12:17 am
- Been upvoted: 1 time
-
Jesse H 2L
- Posts: 58
- Joined: Fri Aug 09, 2019 12:17 am
- Been upvoted: 1 time
Re: Nernst
Brandon Tao 1K wrote:What is nernst equation?
the equation developed by Nernst E=E with the little degree circle - RT/nF nlQ
Who is online
Users browsing this forum: No registered users and 4 guests

