Energetically favorable
Moderators: Chem_Mod, Chem_Admin
-
Hee Sang Kim 3L
- Posts: 33
- Joined: Fri Sep 25, 2015 3:00 am
Energetically favorable
What is energetically favorable and is it the same thing with being exothermic?
Does a energetically favorable reaction not be spontaneous?
Does a energetically favorable reaction not be spontaneous?
-
AlisonWong_1D
- Posts: 6
- Joined: Tue Nov 17, 2015 3:00 am
Re: Energetically favorable
Energetically favorable means no energy input needed for reaction to take place. That means it's spontaneous.
Since there wasn't any energy input, energy was released during the reaction. Therefore, exothermic.
Since there wasn't any energy input, energy was released during the reaction. Therefore, exothermic.
-
Nico Medina
- Posts: 89
- Joined: Wed Sep 30, 2020 9:33 pm
-
Jennifer Fuentes 2K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:17 am
Re: Energetically favorable
Reactions are favorable when they result in a decrease in enthalpy and an increase in entropy of the system. When both of these conditions are met, the reaction occurs naturally. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
-
Lily Rivas 1H
- Posts: 50
- Joined: Mon Jan 03, 2022 9:18 pm
Re: Energetically favorable
A reaction is favorable when the reaction's products have a lot of free energy.
-
elletruchan2I
- Posts: 103
- Joined: Fri Sep 24, 2021 6:46 am
Re: Energetically favorable
Hi!
An energetically favorable equation will be spontaneous. While this mostly occurs with exothermic reactions, it is mostly dependent on the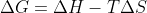 equation. Therefore, a reaction can be spontaneous and endothermic as long as the temperature and deltaS are large enough
equation. Therefore, a reaction can be spontaneous and endothermic as long as the temperature and deltaS are large enough
An energetically favorable equation will be spontaneous. While this mostly occurs with exothermic reactions, it is mostly dependent on the
Re: Energetically favorable
An energetically favorable reaction can refer to exothermic reactions. As a result, these reactions are spontaneous, as. there is no energy input needed for the reaction to take place. Hope that helps!
-
SerenaSabedra
- Posts: 102
- Joined: Fri Sep 24, 2021 7:05 am
Re: Energetically favorable
Energetically favorable just means a reaction has a negative free energy, meaning it's spontaneous because it gives off energy rather than requires it to occur.
-
Gianna Greco 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:54 am
Re: Energetically favorable
Energetically favorable means that no energy input is required for a reaction to occur, so it is spontaneous. If energy is not required for the reaction to occur, then the reaction will release energy and therefore will be exothermic.
-
Benicio Rivera 1F
- Posts: 138
- Joined: Fri Sep 24, 2021 6:42 am
Re: Energetically favorable
We can say that an exothermic reaction is an energetically favorable reaction. If the drive toward lower energy were the only consideration for whether a reaction is able to occur, we would expect that endothermic reactions could never occur spontaneously.
-
Zoe Dhalla 3I
- Posts: 104
- Joined: Fri Sep 24, 2021 5:44 am
Re: Energetically favorable
Since the energy of the system decreases during an exothermic reaction, the products of the system are more stable than the reactants. We can say that an exothermic reaction is an energetically favorable reaction. If a reaction is exothermic ( H is negative) and the entropy S is positive (more disorder), the free energy change is always negative and the reaction is always spontaneous.
Re: Energetically favorable
Energetically favorable reactions do not need any additional help for the reaction to occur meaning they are spontaneous and are exothermic. Thus endothermic reactions are not energetically favorable and thus are not spontaneous.
-
Jeffrey Vo 2A
- Posts: 55
- Joined: Wed Feb 17, 2021 12:19 am
Re: Energetically favorable
When is something is "favorable," it means that that the reaction is spontaneous as Lavelle has stated in his lecture. Although exothermic reactions release energy, it is possible for a reaction to also be endothermic through a higher temperature in the equation of deltaG = deltaH -T*deltaS
-
Alice Weber 3I
- Posts: 94
- Joined: Fri Sep 24, 2021 7:27 am
Re: Energetically favorable
When a reaction is energetically favorable, it is exothermic and spontaneous.
-
WS405590915
- Posts: 52
- Joined: Wed Feb 09, 2022 8:41 pm
Re: Energetically favorable
Jennifer Fuentes 2K wrote:Reactions are favorable when they result in a decrease in enthalpy and an increase in entropy of the system. When both of these conditions are met, the reaction occurs naturally. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
This was really helpful and clarified the topic for me.
Thanks
-
Claire Kim 1F
- Posts: 102
- Joined: Fri Sep 24, 2021 6:27 am
Re: Energetically favorable
Hee Sang Kim 3L wrote:What is energetically favorable and is it the same thing with being exothermic?
Does a energetically favorable reaction not be spontaneous?
I think in a sense, yes. A reaction that is energetically favorable is one that occurs without any outside influence which means that it occurs spontaneously.
Return to “Kinetics vs. Thermodynamics Controlling a Reaction”
Who is online
Users browsing this forum: No registered users and 5 guests

