Rate Constant Units
Moderators: Chem_Mod, Chem_Admin
-
Alexia Joseph 2B
- Posts: 56
- Joined: Thu Jul 27, 2017 3:01 am
Rate Constant Units
How do we tell what the units for the rate constant are based on the order of the reaction?
-
Andres Reynoso 1J
- Posts: 30
- Joined: Fri Sep 29, 2017 7:06 am
Re: Rate Constant Units
You can use the rate law 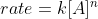 and plug in the units for each variable and simplify.
and plug in the units for each variable and simplify.
For example, for zero order reactions:
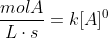
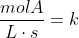
For first order:

)
}=k)
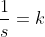
etc. etc...
For example, for zero order reactions:
For first order:
etc. etc...
-
Alyssa Parry Disc 1H
- Posts: 53
- Joined: Sat Jul 22, 2017 3:01 am
Re: Rate Constant Units
Or you could think of it like this units of k=M^(1-n).s-1 where n is the order.
-
David Minasyan 1C
- Posts: 54
- Joined: Thu Jul 13, 2017 3:00 am
Re: Rate Constant Units
Know that zero order reactions are mol/(L*s) and then as you go up one order, multiply by L/mol each time.
-
RussellChin_3A
- Posts: 42
- Joined: Sat Jul 22, 2017 3:01 am
Re: Rate Constant Units
The units of K must be able to cancel out with the units of the concentration in order for the rate units to always be M/s. so just add on however many M/s needed in order to get the rate to its proper units
-
Belle Calforda3f
- Posts: 67
- Joined: Fri Sep 29, 2017 7:07 am
Re: Rate Constant Units
using either the integrated rate law or the half life eqn can help you figure out the units for the rate constant without having to memorize anything. If you plug in the units for everything but k, you can figure out what k has to be for the equations to work.
-
Erik Khong 2E
- Posts: 50
- Joined: Fri Sep 29, 2017 7:07 am
-
David Zhou 1L
- Posts: 61
- Joined: Fri Sep 29, 2017 7:04 am
Re: Rate Constant Units
Remember that multiplying my L/mol is the same thing as dividing by M, or multiplying by M-1.
-
William Xu Dis 1D
- Posts: 31
- Joined: Fri Sep 29, 2017 7:05 am
Re: Rate Constant Units
Also, when solving for k through the equations, the units should cancel out to the proper units anyway, but it is useful to know what the units are for different orders anyway.
-
Michael Downs 1L
- Posts: 29
- Joined: Thu Jul 13, 2017 3:00 am
Return to “Kinetics vs. Thermodynamics Controlling a Reaction”
Who is online
Users browsing this forum: No registered users and 6 guests

