Activation Energy
Moderators: Chem_Mod, Chem_Admin
-
Jessica Helfond 2F
- Posts: 60
- Joined: Fri Sep 28, 2018 12:16 am
-
Vincent Li 4L
- Posts: 48
- Joined: Fri Sep 28, 2018 12:19 am
Re: Activation Energy
The dependence of k on temperature and activation energy can be seen in the Arrhenius equation, given as 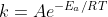 . The constant A, is a parameter found through experimentation, similar to the activation energy Ea. As you can see, as the activation energy increases, k approaches 0. As k decreases, rate decreases as they are proportional to each other. As temperature increases, the value of k approaches its maximum, which then increases rate. Hope this helps, but I believe we'll be going more in-depth with the equation some time next week probably.
. The constant A, is a parameter found through experimentation, similar to the activation energy Ea. As you can see, as the activation energy increases, k approaches 0. As k decreases, rate decreases as they are proportional to each other. As temperature increases, the value of k approaches its maximum, which then increases rate. Hope this helps, but I believe we'll be going more in-depth with the equation some time next week probably.
-
Karan Thaker 2L
- Posts: 75
- Joined: Fri Sep 28, 2018 12:26 am
Re: Activation Energy
That's exactly how I think of it as well. Simply examine the equation and see what would happen. With the Arrhenius equation, it can be a little confusing to predict it right off the bat so plug in numbers to see what happens as well.
Return to “Kinetics vs. Thermodynamics Controlling a Reaction”
Who is online
Users browsing this forum: No registered users and 9 guests

