Can anyone help me start this problem or tell me which lecture to find the material?
Dinitrogen pentoxide, N2O5, decomposes by first‑order kinetics with a rate constant of 3.7×10−5 s−1 at 298 K.
What is the half‑life, in hours, of N2O5 at 298 K?
Thanks!
Sapling #11
Moderators: Chem_Mod, Chem_Admin
-
Jonathan Haimowitz 3B
- Posts: 56
- Joined: Wed Nov 18, 2020 12:28 am
- Been upvoted: 2 times
Re: Sapling #11
The question states that this decomposition is first-order, so use the first-order half life equation: 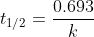
Note that it asks for the half-life in hours, and the result of this equation will be in seconds.
Note that it asks for the half-life in hours, and the result of this equation will be in seconds.
Re: Sapling #11
Hi, all the information on half-life equations is given in today's lecture, lecture 23. The rate constant you are given is k and it is a first-order reaction so you want to use the equation for a first-order half-life which is ln2/k. Be careful of units.
-
Daniela Santana 2L
- Posts: 103
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Sapling #11
Hi! In order to solve for the half life of something you use the equation t1/2 = ln2/k. ln2 approximately equals .693 so solve for .693/3.7x10^-5. The value you get from this will be in seconds so be sure to convert this value to hours by applying 60 as many times needed. I hope this helps!!
Return to “Kinetics vs. Thermodynamics Controlling a Reaction”
Who is online
Users browsing this forum: No registered users and 6 guests

