b) Does kinetic control predominate at low or high temperatures?
I looked up kinetic vs thermodynamic control, and the answer was explained with this graph:
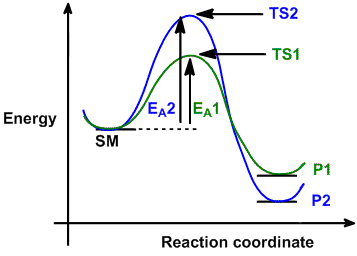
Why is it that a lower activation energy corresponds to a higher free energy of the products? The answer to part b is that kinetic control predominates at lower temperatures.
Edit: After thinking about it I think that this graph corresponds to reaction pathways where one pathway is thermodynamically favored and the other one is kinetically favored, and only under these conditions will you have thermodynamic/kinetic control. In other reactions where for one pathway the Ea and the free energy of the products are both lower, then there is no control of one vs the other and that pathway will always be favored at any temperature. If anyone could confirm/refute this thank you :)

