Hello,
I am a little uncertain about catalysts and rate laws. I understand they do not alter the rate constant in a zero order reaction as seen in the course reader but do they affect rate laws in higher orders?
Thanks in advance.
Catalyst
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Catalyst
Yes, catalysts should affect the rate of a reaction. Catalysts speed up the reaction by lowering the activation energy of that reaction. As shown by the Arrhenius equation, lowering the activation energy 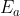 increases the rate constant, k.
increases the rate constant, k.
-
Wenqian_Deng_1L
- Posts: 23
- Joined: Wed Sep 21, 2016 3:00 pm
Re: Catalyst
Does changing the temperature also affect the catalyst, which then affects the activation energy and the rate constant?
Re: Catalyst
Increasing temperature will increase the rate of reaction with catalysts, but up to a point. At around 40 degrees C, some enzymes will denature and no longer be able to catalyze.
Who is online
Users browsing this forum: No registered users and 9 guests

