15.3(c)
Moderators: Chem_Mod, Chem_Admin
-
Jasmine Botello 2F
- Posts: 67
- Joined: Fri Sep 29, 2017 7:04 am
15.3(c)
For this part of the question, it asks "What is the unique rate of the reaction" and I am not really sure what it is asking me to find..?
-
Kate Zeile 2D
- Posts: 39
- Joined: Sat Jul 22, 2017 3:01 am
Re: 15.3(c)
In the textbook, the unique average reaction rate for reaction 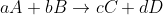 is :
is :
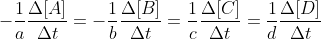
In problem 15.3, the unique rate of the reaction refers to the unique rate of the reaction of 2NO2 (the reactant in the equation). Therefore, the unique rate of the reaction would be -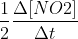 . You would divide by 2 since the coefficient of NO2 is 2 in the given reaction.
. You would divide by 2 since the coefficient of NO2 is 2 in the given reaction.
In problem 15.3, the unique rate of the reaction refers to the unique rate of the reaction of 2NO2 (the reactant in the equation). Therefore, the unique rate of the reaction would be -
-
Kate Zeile 2D
- Posts: 39
- Joined: Sat Jul 22, 2017 3:01 am
Re: 15.3(c)
Sorry I would like to make a correction. The unique rate of the reaction refers to any one of the parts of this equation: 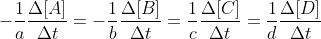 ; all are equal. However, since the concentration of NO2 is given in the problem, we use that part. The unique rate of the reaction is the concentration that is given for one part of the chemical equation divided by its coefficient (and a negative if it is a reactant).
; all are equal. However, since the concentration of NO2 is given in the problem, we use that part. The unique rate of the reaction is the concentration that is given for one part of the chemical equation divided by its coefficient (and a negative if it is a reactant).
Who is online
Users browsing this forum: No registered users and 10 guests

